Page 172 - Handbook of Battery Materials
P. 172
4.4 Conversion of EMD to LiMnO 2 or LiMn 2 O 4 for Rechargeable Li Batteries 141
a
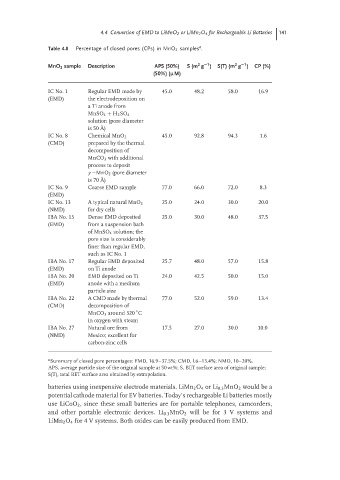
Table 4.8 Percentage of closed pores (CPs) in MnO 2 samples .
−1
2
−1
2
MnO 2 sample Description APS (50%) S (m g ) S(T) (m g ) CP (%)
(50%) (µM)
IC No. 1 Regular EMD made by 45.0 48.2 58.0 16.9
(EMD) the electrodeposition on
a Ti anode from
MnSO 4 + H 2 SO 4
solution (pore diameter
is 50 ˚ A)
IC No. 8 Chemical MnO 2 45.0 92.8 94.3 1.6
(CMD) prepared by the thermal
decomposition of
MnCO 3 with additional
process to deposit
γ −MnO 2 (pore diameter
is 70 ˚ A)
IC No. 9 Coarse EMD sample 77.0 66.0 72.0 8.3
(EMD)
IC No. 13 A typical natural MnO 2 25.0 24.0 30.0 20.0
(NMD) for dry cells
IBA No. 15 Dense EMD deposited 25.0 30.0 48.0 37.5
(EMD) from a suspension bath
of MnSO 4 solution; the
pore size is considerably
finer than regular EMD,
such as IC No. 1
IBA No. 17 Regular EMD deposited 25.7 48.0 57.0 15.8
(EMD) on Ti anode
IBA No. 20 EMD deposited on Ti 24.0 42.5 50.0 15.0
(EMD) anode with a medium
particle size
IBA No. 22 A CMD made by thermal 77.0 52.0 59.0 13.4
(CMD) decomposition of
◦
MnCO 3 around 320 C
in oxygen with steam
IBA No. 27 Natural ore from 17.5 27.0 30.0 10.0
(NMD) Mexico; excellent for
carbon-zinc cells
a Summary of closed pore percentages: EMD, 16.9–37.5%; CMD, l.6–13.4%; NMD, 10–20%.
APS, average particle size of the original sample at 50 wt%; S, BET surface area of original sample;
S(T), total BET surface area obtained by extrapolation.
batteries using inexpensive electrode materials. LiMn 2 O 4 or Li 0.3 MnO 2 would be a
potential cathode material for EV batteries. Today’s rechargeable Li batteries mostly
use LiCoO 2 , since these small batteries are for portable telephones, camcorders,
and other portable electronic devices. Li 0.3 MnO 2 will be for 3 V systems and
LiMn 2 O 4 for 4 V systems. Both oxides can be easily produced from EMD.