Page 167 - Handbook of Battery Materials
P. 167
136 4 Electrochemistry of Manganese Oxides
−
MnO + H O + e = MnOOH + OH MnOOH + H O + e
2
2
2
0 = Mn(OH) + OH −
2
Potential, V(vs. HgO/Hg) −0.3 : : : 3M KOH, N gas
2
3M KOH, in air
6M KOH, N gas
2
:
9M KOH, N gas
2
9M KOH, in air
:
−0.6 IC 17 : 100 mg, TAB3: 30 mg
Discharge rate: 3 mA (30 mA/g)
0 10 20 30 40
mAH
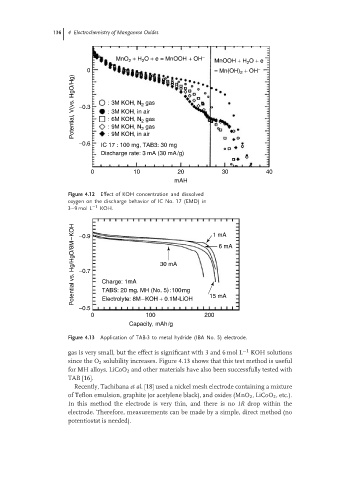
Figure 4.12 Effect of KOH concentration and dissolved
oxygen on the discharge behavior of IC No. 17 (EMD) in
3–9 mol L −1 KOH. 1 mA
Potential vs. Hg/HgO/8M–KOH −0.7 Charge: 1mA 30 mA 6 mA
−0.9
Electrolyte: 8M–KOH + 0.1M-LiOH
−0.5 TABS: 20 mg. MH (No. 5):100mg 15 mA
0 100 200
Capacity, mAh/g
Figure 4.13 Application of TAB-3 to metal hydride (IBA No. 5) electrode.
gas is very small, but the effect is significant with 3 and 6 mol L −1 KOH solutions
since the O 2 solubility increases. Figure 4.13 shows that this test method is useful
for MH alloys. LiCoO 2 and other materials have also been successfully tested with
TAB [16].
Recently, Tachibana et al. [18] used a nickel mesh electrode containing a mixture
of Teflon emulsion, graphite (or acetylene black), and oxides (MnO 2 ,LiCoO 2 , etc.).
In this method the electrode is very thin, and there is no IR drop within the
electrode. Therefore, measurements can be made by a simple, direct method (no
potentiostat is needed).