Page 162 - Handbook of Battery Materials
P. 162
4.2 Electrochemical Properties of EMD 131
MnO Potential MnOOH
2
b'
E x E ο ο
E i i E i E = E = E
i
m
b
E (= E ο) c
m
Potential b ∆F = nFE c Potential ∆F = nF E ο
ο
= a b c d
a b c d
a d a d
0 50 100 0 50 (Ag) 100%
Reduction (%) 100 50 (Ag O) 0%
2
Composition
(A) One - Phase Redox System (B) Two - Phase Redox System
(MnO , V O , LiMn O etc) (PbO , Ag O, HgO, Zn, Pb, etc)
2
2
2
2
4
2
5
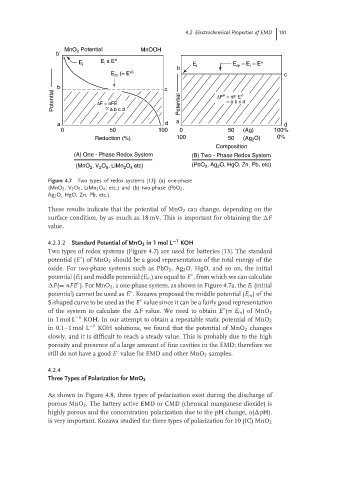
Figure 4.7 Two types of redox systems [13]: (a) one-phase
(MnO 2 ,V 2 O 5 ,LiMn 2 O 4 , etc.) and (b) two-phase (PbO 2 ,
Ag 2 O, HgO, Zn, Pb, etc.).
These results indicate that the potential of MnO 2 can change, depending on the
surface condition, by as much as 18 mV. This is important for obtaining the F
value.
4.2.3.2 Standard Potential of MnO 2 in 1 mol L −1 KOH
Two types of redox systems (Figure 4.7) are used for batteries [13]. The standard
◦
potential (E )ofMnO 2 should be a good representation of the total energy of the
oxide. For two-phase systems such as PbO 2 ,Ag 2 O, HgO, and so on, the initial
potential (E i ) and middle potential (E m )areequalto E , from which we can calculate
◦
◦
F(= nFE ). For MnO 2 , a one-phase system, as shown in Figure 4.7a, the E i (initial
◦
potential) cannot be used as E . Kozawa proposed the middle potential (E m )ofthe
S-shaped curve to be used as the E value since it can be a fairly good representation
◦
◦
of the system to calculate the F value. We need to obtain E (= E m )ofMnO 2
in 1 mol L −1 KOH. In our attempt to obtain a repeatable static potential of MnO 2
in 0.1–1 mol L −1 KOH solutions, we found that the potential of MnO 2 changes
slowly, and it is difficult to reach a steady value. This is probably due to the high
porosity and presence of a large amount of fine cavities in the EMD; therefore we
◦
still do not have a good E value for EMD and other MnO 2 samples.
4.2.4
Three Types of Polarization for MnO 2
As shown in Figure 4.8, three types of polarization exist during the discharge of
porous MnO 2 . The battery active EMD or CMD (chemical manganese dioxide) is
highly porous and the concentration polarization due to the pH change, η( pH),
is very important. Kozawa studied the three types of polarization for 10 (IC) MnO 2