Page 159 - Handbook of Battery Materials
P. 159
128 4 Electrochemistry of Manganese Oxides
x in MnO x x in MnO x
20 18 16 14 12 10 20 18 16 14 12 10
+02 +02
STORAGE TEMP at 12 mA - h
1 2 010 mA 0 1, 2, 3 a 1 2 3 23 °C
12 mA - h
0 °C
100 mA
CCV (IR - free) vs Hg/HgO (9M KOH), volt −02 I II 2 CCV (IR - free) vs Hg/HgO (9M KOH), volt −02 I II
0
45 °C
−04
−04
I
III
−06
IV −06 III 3 2
IV
−08 −08
0 10 20 30 40 50 0 10 20 30 40 50
(a) DISCHARGE, mA - h (b) DISCHARGE, mA - h
x in MnO x x in MnO x
20 18 16 14 12 10 20 18 16 14 12 10
+02 +02
STORAGE TEMP at 16 mA - h
STORAGE TEMP at 20 mA - h
16 mA - h a 1 2 3 23 °C 0 1, 2, 3 20 mA - h 1 2 23 °C
0 °C
CCV (IR - free) vs Hg/HgO (9M KOH), volt −02 I b 3 II CCV (IR - free) vs Hg/HgO (9M KOH), volt −02 I a b II 3 45 °C
0 °C
0
1, 2, 3
45 °C
−04
−04
3
2
2
−06
−06
III
III
IV
IV
−08 −08
0 10 20 30 40 50 0 10 20 30 40 50
(c) DISCHARGE, mA - h (d) DISCHARGE, mA - h
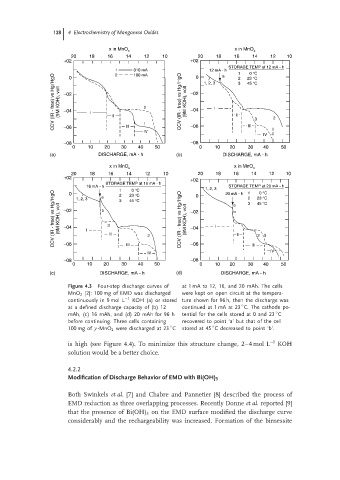
Figure 4.3 Four-step discharge curves of at 1 mA to 12, 16, and 20 mAh. The cells
MnO 2 [2]: 100 mg of EMD was discharged were kept on open circuit at the tempera-
continuously in 9 mol L −1 KOH (a) or stored ture shown for 96 h, then the discharge was
◦
at a defined discharge capacity of (b) 12 continued at 1 mA at 23 C. The cathode po-
◦
mAh, (c)16mAh,and(d)20mAh for96h tential for the cells stored at 0 and 23 C
before continuing. Three cells containing recovered to point ‘a’ but that of the cell
◦
◦
100 mg of γ -MnO 2 were discharged at 23 C stored at 45 C decreased to point ‘b’.
is high (see Figure 4.4). To minimize this structure change, 2–4 mol L −l KOH
solution would be a better choice.
4.2.2
Modification of Discharge Behavior of EMD with Bi(OH) 3
Both Swinkels et al. [7] and Chabre and Pannetier [8] described the process of
EMD reduction as three overlapping processes. Recently Donne et al. reported [9]
that the presence of Bi(OH) 3 on the EMD surface modified the discharge curve
considerably and the rechargeability was increased. Formation of the birnessite