Page 161 - Handbook of Battery Materials
P. 161
130 4 Electrochemistry of Manganese Oxides
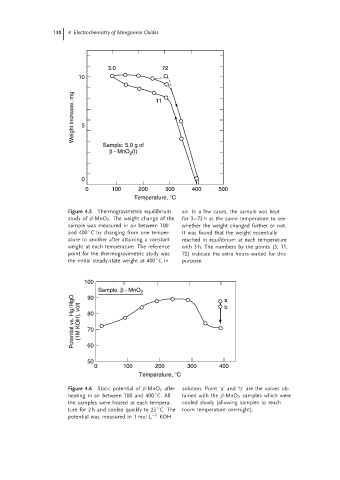
3.0 72
10
Weight increase, mg 5 11
Sample: 5.0 g of
(I)
β - MnO 2
0
0 100 200 300 400 500
Temperature, °C
Figure 4.5 Thermogravimetric equilibrium air. In a few cases, the sample was kept
study of β-MnO 2 . The weight change of the for 3–72 h at the same temperature to see
sample was measured in air between 100 whether the weight changed further or not.
◦
and 400 C by changing from one temper- It was found that the weight essentially
ature to another after attaining a constant reached in equilibrium at each temperature
weight at each temperature. The reference with 3 h. The numbers by the points (3, 11,
point for the thermogravimetric study was 72) indicate the extra hours waited for this
◦
the initial steady-state weight at 400 Cin purpose.
100
Sample: β - MnO 2 a
Potential vs. Hg/HgO (1M KOH), volt 80 b
90
70
60
50
0 100 200 300 400
Temperature, °C
Figure 4.6 Static potential of β-MnO 2 after solution. Point ‘a’ and ‘b’ are the values ob-
◦
heating in air between 100 and 400 C. All tained with the β-MnO 2 samples which were
the samples were heated at each tempera- cooled slowly (allowing samples to reach
◦
ture for 2 h and cooled quickly to 25 C. The room temperature overnight).
potential was measured in 1 mol L −1 KOH