Page 158 - Handbook of Battery Materials
P. 158
4.2 Electrochemical Properties of EMD 127
1.5
a
A: 9M KOH
b c d
Potential of MnO 2 nO 2 vs Zn Electrode 1.0 MnO 2 R 1 MnO OH 4 2 Mn (OH) 2
e
1.5
B: 5M NH Cl + 2M ZnCl
a'
1.0 R b' c'
R 2 e'
MnO 2 MnO 1.5 MnO
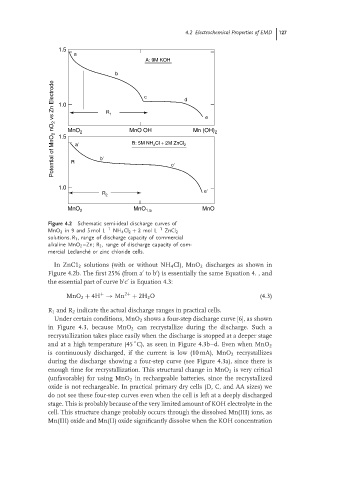
Figure 4.2 Schematic semi-ideal discharge curves of
MnO 2 in 9 and 5 mol L −1 NH 4 Cl 2 + 2mol L −1 ZnCl 2
solutions. R 1 , range of discharge capacity of commercial
alkaline MnO 2 –Zn; R 2 , range of discharge capacity of com-
mercial Leclanch´ e or zinc chloride cells.
In ZnC1 2 solutions (with or without NH 4 Cl), MnO 2 discharges as shown in
Figure 4.2b. The first 25% (from a to b ) is essentially the same Equation 4. , and
the essential part of curve b c is Equation 4.3:
+
MnO 2 + 4H → Mn 2+ + 2H 2 O (4.3)
R 1 and R 2 indicate the actual discharge ranges in practical cells.
Under certain conditions, MnO 2 shows a four-step discharge curve [6], as shown
in Figure 4.3, because MnO 2 can recrystallize during the discharge. Such a
recrystallization takes place easily when the discharge is stopped at a deeper stage
◦
and at a high temperature (45 C), as seen in Figure 4.3b–d. Even when MnO 2
is continuously discharged, if the current is low (10 mA), MnO 2 recrystallizes
during the discharge showing a four-step curve (see Figure 4.3a), since there is
enough time for recrystallization. This structural change in MnO 2 is very critical
(unfavorable) for using MnO 2 in rechargeable batteries, since the recrystallized
oxide is not rechargeable. In practical primary dry cells (D, C, and AA sizes) we
do not see these four-step curves even when the cell is left at a deeply discharged
stage. This is probably because of the very limited amount of KOH electrolyte in the
cell. This structure change probably occurs through the dissolved Mn(III) ions, as
Mn(III) oxide and Mn(II) oxide significantly dissolve when the KOH concentration