Page 160 - Handbook of Battery Materials
P. 160
4.2 Electrochemical Properties of EMD 129
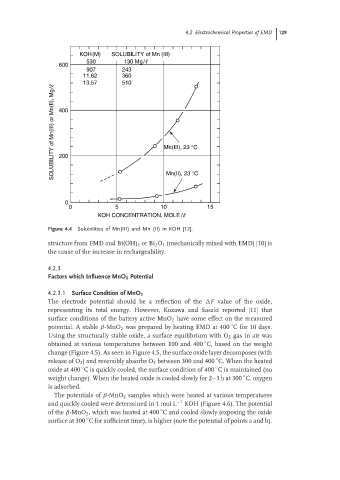
KOH(M) SOLUBILITY of Mn (III)
530 130 Mg/
600
907 243
11.62 360
13.57 510
SOLUBILITY of Mn(III) or Mn(II), Mg/ 400 Mn(III), 23 °C
200
0 Mn(II), 23 °C
0 5 10 15
KOH CONCENTRATION, MOLE /
Figure 4.4 Solubilities of Mn(III) and Mn (II) in KOH [12].
structure from EMD and Bi(OH) 3 or Bi 2 O 3 (mechanically mixed with EMD) [10] is
the cause of the increase in rechargeability.
4.2.3
Factors which Influence MnO 2 Potential
4.2.3.1 Surface Condition of MnO 2
The electrode potential should be a reflection of the F value of the oxide,
representing its total energy. However, Kozawa and Sasaki reported [11] that
surface conditions of the battery active MnO 2 have some effect on the measured
potential. A stable β-MnO 2 was prepared by heating EMD at 400 Cfor 10 days.
◦
Using the structurally stable oxide, a surface equilibrium with O 2 gas in air was
◦
obtained at various temperatures between 100 and 400 C, based on the weight
change (Figure 4.5). As seen in Figure 4.5, the surface oxide layer decomposes (with
◦
release of O 2 ) and reversibly absorbs O 2 between 300 and 400 C. When the heated
◦
◦
oxide at 400 C is quickly cooled, the surface condition of 400 C is maintained (no
◦
weight change). When the heated oxide is cooled slowly for 2–3 h at 300 C, oxygen
is adsorbed.
The potentials of β-MnO 2 samples which were heated at various temperatures
and quickly cooled were determined in 1 mol L −1 KOH (Figure 4.6). The potential
◦
of the β-MnO 2 , which was heated at 400 C and cooled slowly (exposing the oxide
◦
surface at 300 C for sufficient time), is higher (note the potential of points a and b).