Page 157 - Handbook of Battery Materials
P. 157
126 4 Electrochemistry of Manganese Oxides
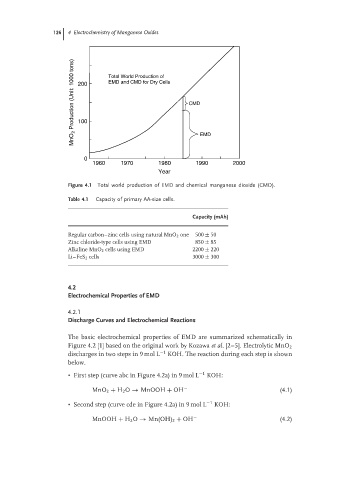
MnO 2 Production (Unit: 1000 tons) 200 Total World Production of CMD
EMD and CMD for Dry Cells
100
0 EMD
1960 1970 1980 1990 2000
Year
Figure 4.1 Total world production of EMD and chemical manganese dioxide (CMD).
Table 4.1 Capacity of primary AA-size cells.
Capacity (mAh)
Regular carbon–zinc cells using natural MnO 2 one 500 ± 50
Zinc chloride-type cells using EMD 850 ± 85
Alkaline MnO 2 cells using EMD 2200 ± 220
Li–FeS 2 cells 3000 ± 300
4.2
Electrochemical Properties of EMD
4.2.1
Discharge Curves and Electrochemical Reactions
The basic electrochemical properties of EMD are summarized schematically in
Figure 4.2 [1] based on the original work by Kozawa et al. [2–5]. Electrolytic MnO 2
discharges in two steps in 9 mol L −1 KOH. The reaction during each step is shown
below.
• First step (curve abc in Figure 4.2a) in 9 mol L −1 KOH:
MnO 2 + H 2 O → MnOOH + OH − (4.1)
• Second step (curve cde in Figure 4.2a) in 9 mol L −1 KOH:
MnOOH + H 2 O → Mn(OH) 2 + OH − (4.2)