Page 171 - Handbook of Battery Materials
P. 171
140 4 Electrochemistry of Manganese Oxides
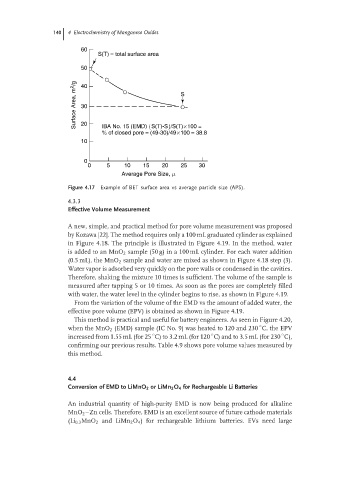
60
S(T) = total surface area
50
Surface Area, m 2 /g 30 S
40
20
IBA No. 15 (EMD) {S(T)-S}/S(T)×100 =
% of closed pore = (49-30)/49×100 = 38.8
10
0
0 5 10 15 20 25 30
Average Pore Size, m
Figure 4.17 Example of BET surface area vs average particle size (APS).
4.3.3
Effective Volume Measurement
A new, simple, and practical method for pore volume measurement was proposed
by Kozawa [22]. The method requires only a 100 mL graduated cylinder as explained
in Figure 4.18. The principle is illustrated in Figure 4.19. In the method, water
is added to an MnO 2 sample (50 g) in a 100 mL cylinder. For each water addition
(0.5 mL), the MnO 2 sample and water are mixed as shown in Figure 4.18 step (3).
Water vapor is adsorbed very quickly on the pore walls or condensed in the cavities.
Therefore, shaking the mixture 10 times is sufficient. The volume of the sample is
measured after tapping 5 or 10 times. As soon as the pores are completely filled
with water, the water level in the cylinder begins to rise, as shown in Figure 4.19.
From the variation of the volume of the EMD vs the amount of added water, the
effective pore volume (EPV) is obtained as shown in Figure 4.19.
This method is practical and useful for battery engineers. As seen in Figure 4.20,
◦
when the MnO 2 (EMD) sample (IC No. 9) was heated to 120 and 230 C, the EPV
◦
◦
◦
increased from 1.55 mL (for 25 C) to 3.2 mL (for 120 C) and to 3.5 mL (for 230 C),
confirming our previous results. Table 4.9 shows pore volume values measured by
this method.
4.4
Conversion of EMD to LiMnO 2 or LiMn 2 O 4 for Rechargeable Li Batteries
An industrial quantity of high-purity EMD is now being produced for alkaline
MnO 2 –Zn cells. Therefore, EMD is an excellent source of future cathode materials
(Li 0.3 MnO 2 and LiMn 2 O 4 ) for rechargeable lithium batteries. EVs need large