Page 273 - Handbook of Battery Materials
P. 273
9.2 Theory and Basic Principles 243
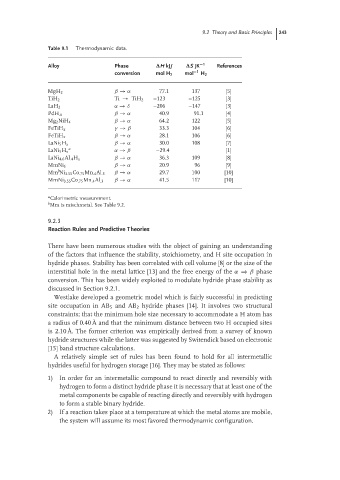
Table 9.1 Thermodynamic data.
Alloy Phase ∆H kJ/ ∆S JK −1 References
conversion mol H 2 mol −1 H 2
MgH 2 β → α 77.1 137 [5]
TiH 2 Ti → TiH 2 –123 –125 [3]
α → δ –206 –147 [3]
LaH 2
PdH .6 β → α 40.9 91.1 [4]
Mg 2 NiH 4 β → α 64.2 122 [5]
FeTiH x γ → β 33.3 104 [6]
FeTiH x β → α 28.1 106 [6]
LaNi 5 H x β → α 30.0 108 [7]
a
LaNi 5 H x α → β –29.4 [1]
LaNi 4.6 Al .4 H x β → α 36.3 109 [8]
MmNi 5 β → α 20.9 96 [9]
b
Mm Ni 3.55 Co .75 Mn .4 Al .3 β → α 29.7 100 [10]
MmNi 3.55 Co .75 Mn .4 Al .3 β → α 41.5 117 [10]
a
Calorimetric measurement.
b Mm is mischmetal. See Table 9.2.
9.2.3
Reaction Rules and Predictive Theories
There have been numerous studies with the object of gaining an understanding
of the factors that influence the stability, stoichiometry, and H site occupation in
hydride phases. Stability has been correlated with cell volume [8] or the size of the
interstitial hole in the metal lattice [13] and the free energy of the α ⇒ β phase
conversion. This has been widely exploited to modulate hydride phase stability as
discussed in Section 9.2.1.
Westlake developed a geometric model which is fairly successful in predicting
site occupation in AB 5 and AB 2 hydride phases [14]. It involves two structural
constraints; that the minimum hole size necessary to accommodate a H atom has
a radius of 0.40 ˚ A and that the minimum distance between two H occupied sites
is 2.10 ˚ A. The former criterion was empirically derived from a survey of known
hydride structures while the latter was suggested by Switendick based on electronic
[15] band structure calculations.
A relatively simple set of rules has been found to hold for all intermetallic
hydrides useful for hydrogen storage [16]. They may be stated as follows:
1) In order for an intermetallic compound to react directly and reversibly with
hydrogen to form a distinct hydride phase it is necessary that at least one of the
metal components be capable of reacting directly and reversibly with hydrogen
to form a stable binary hydride.
2) If a reaction takes place at a temperature at which the metal atoms are mobile,
the system will assume its most favored thermodynamic configuration.