Page 275 - Handbook of Battery Materials
P. 275
9.3 Metal Hydride–Nickel Batteries 245
a
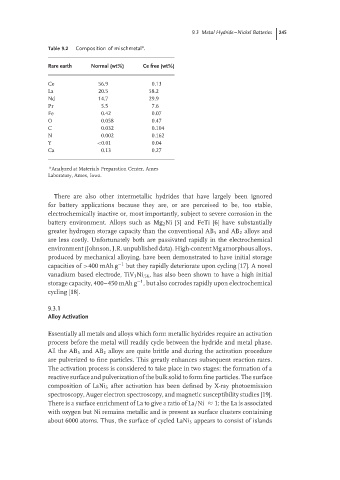
Table 9.2 Composition of mischmetal .
Rare earth Normal (wt%) Ce free (wt%)
Ce 56.9 0.13
La 20.5 58.2
Nd 14.7 29.9
Pr 5.5 7.6
Fe 0.42 0.07
O 0.058 0.47
C 0.032 0.104
N 0.002 0.162
Y <0.01 0.04
Ca 0.13 0.27
a
Analyzed at Materials Preparation Center, Ames
Laboratory, Ames, Iowa.
There are also other intermetallic hydrides that have largely been ignored
for battery applications because they are, or are perceived to be, too stable,
electrochemically inactive or, most importantly, subject to severe corrosion in the
battery environment. Alloys such as Mg 2 Ni [5] and FeTi [6] have substantially
greater hydrogen storage capacity than the conventional AB 5 and AB 2 alloys and
are less costly. Unfortunately both are passivated rapidly in the electrochemical
environment (Johnson, J.R. unpublished data). High-content Mg amorphous alloys,
produced by mechanical alloying, have been demonstrated to have initial storage
capacities of >400 mAh g −1 but they rapidly deteriorate upon cycling [17]. A novel
vanadium based electrode, TiV 3 Ni .56 , has also been shown to have a high initial
−1
storage capacity, 400–450 mAh g , but also corrodes rapidly upon electrochemical
cycling [18].
9.3.1
Alloy Activation
Essentially all metals and alloys which form metallic hydrides require an activation
process before the metal will readily cycle between the hydride and metal phase.
All the AB 5 and AB 2 alloys are quite brittle and during the activation procedure
are pulverized to fine particles. This greatly enhances subsequent reaction rates.
The activation process is considered to take place in two stages: the formation of a
reactive surface and pulverization of the bulk solid to form fine particles. The surface
composition of LaNi 5 after activation has been defined by X-ray photoemission
spectroscopy, Auger electron spectroscopy, and magnetic susceptibility studies [19].
There is a surface enrichment of La to give a ratio of La/Ni ≈ 1; the La is associated
with oxygen but Ni remains metallic and is present as surface clusters containing
about 6000 atoms. Thus, the surface of cycled LaNi 5 appears to consist of islands