Page 278 - Handbook of Battery Materials
P. 278
248 9 Metal Hydride Electrodes
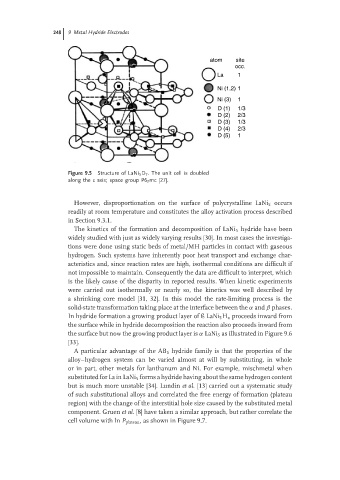
atom site
occ.
La 1
Ni (1,2) 1
Ni (3) 1
D (1) 1/3
D (2) 2/3
D (3) 1/3
D (4) 2/3
D (5) 1
Figure 9.5 Structure of LaNi 5 D 7 . The unit cell is doubled
along the c axis; space group P6 3 mc [27].
However, disproportionation on the surface of polycrystalline LaNi 5 occurs
readily at room temperature and constitutes the alloy activation process described
in Section 9.3.1.
The kinetics of the formation and decomposition of LaNi 5 hydride have been
widely studied with just as widely varying results [30]. In most cases the investiga-
tions were done using static beds of metal/MH particles in contact with gaseous
hydrogen. Such systems have inherently poor heat transport and exchange char-
acteristics and, since reaction rates are high, isothermal conditions are difficult if
not impossible to maintain. Consequently the data are difficult to interpret, which
is the likely cause of the disparity in reported results. When kinetic experiments
were carried out isothermally or nearly so, the kinetics was well described by
a shrinking core model [31, 32]. In this model the rate-limiting process is the
solid-state transformation taking place at the interface between the α and β phases.
In hydride formation a growing product layer of ß LaNi 5 H x proceeds inward from
the surface while in hydride decomposition the reaction also proceeds inward from
the surface but now the growing product layer is α LaNi 5 as illustrated in Figure 9.6
[33].
A particular advantage of the AB 5 hydride family is that the properties of the
alloy–hydrogen system can be varied almost at will by substituting, in whole
or in part, other metals for lanthanum and Ni. For example, mischmetal when
substituted for La in LaNi 5 forms a hydride having about the same hydrogen content
but is much more unstable [34]. Lundin et al. [13] carried out a systematic study
of such substitutional alloys and correlated the free energy of formation (plateau
region) with the change of the interstitial hole size caused by the substituted metal
component. Gruen et al. [8] have taken a similar approach, but rather correlate the
cell volume with ln P plateau , as shown in Figure 9.7.