Page 281 - Handbook of Battery Materials
P. 281
9.3 Metal Hydride–Nickel Batteries 251
4
MmB 5
∆H = -29.7(2.2) kJ/mol H 2
T
3 ∆S = -100(7) J/K mol H 2
2
LnP 30 LaNi 5
1
0 ∆H = -41.5(0.8) kJ/mol H 2
T
∆S = -117(2) J/K mol H 2
Cefree Mm*B 5
-1
2.6 2.7 2.8 2.9 3.0 3.1 3.2 3.3 3.4
1000/T
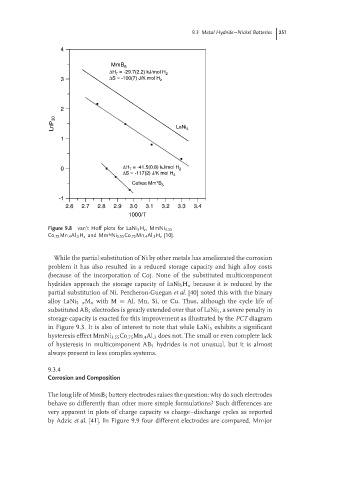
Figure 9.8 van’t Hoff plots for LaNi 5 H x ,MmNi 3.55
Co .75 Mn .4 Al .3 H x and Mm*Ni 3.55 Co .75 Mn .4 Al .3 H x [10].
While the partial substitution of Ni by other metals has ameliorated the corrosion
problem it has also resulted in a reduced storage capacity and high alloy costs
(because of the incorporation of Co). None of the substituted multicomponent
hydrides approach the storage capacity of LaNi 5 H x because it is reduced by the
partial substitution of Ni. Percheron-Guegan et al. [40] noted this with the binary
alloy LaNi 5−x M x with M = Al, Mn, Si, or Cu. Thus, although the cycle life of
substituted AB 5 electrodes is greatly extended over that of LaNi 5 , a severe penalty in
storage capacity is exacted for this improvement as illustrated by the PCT diagram
in Figure 9.3. It is also of interest to note that while LaNi 5 exhibits a significant
hysteresis effect MmNi 3.55 Co .75 Mn .4 Al .3 does not. The small or even complete lack
of hysteresis in multicomponent AB 5 hydrides is not unusual, but it is almost
always present in less complex systems.
9.3.4
Corrosion and Composition
ThelonglifeofMmB 5 battery electrodes raises the question: why do such electrodes
behave so differently than other more simple formulations? Such differences are
very apparent in plots of charge capacity vs charge–discharge cycles as reported
by Adzic et al. [41]. In Figure 9.9 four different electrodes are compared, Mm(or