Page 277 - Handbook of Battery Materials
P. 277
9.3 Metal Hydride–Nickel Batteries 247
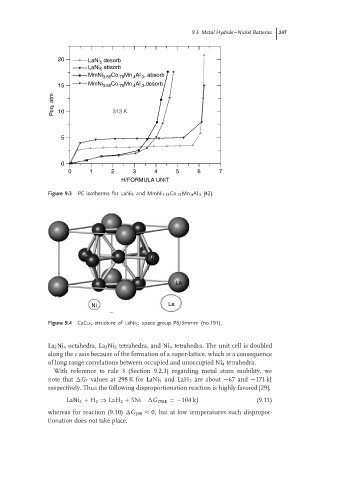
20 LaNi 5 desorb
LaNi 5 absorb
MmNi 3.55 Co .75 Mn .4 Al .3 , absorb
15 MmNi 3.55 Co .75 Mn .4 Al .3 ,desorb
Peq, atm 10 313 K
5
0
0 1 2 3 4 5 6 7
H/FORMULA UNIT
Figure 9.3 PC isotherms for LaNi 5 and MmNi 3.55 Co .75 Mn .4 Al .3 [42].
Ni
N
i
La
Ni La
Figure 9.4 CaCu 5 structure of LaNi 5 ; space group P6/3mmm (no.191).
La 2 Ni 4 octahedra, La 2 Ni 2 tetrahedra, and Ni 4 tetrahedra. The unit cell is doubled
along the c axis because of the formation of a super-lattice, which is a consequence
of long range correlations between occupied and unoccupied Ni 4 tetrahedra.
With reference to rule 3 (Section 9.2.3) regarding metal atom mobility, we
note that G f values at 298 K for LaNi 5 and LaH 2 are about −67 and −171 kJ
respectively. Thus the following disproportionation reaction is highly favored [29],
LaNi 5 + H 2 ⇒ LaH 2 + 5Ni G 298K =−104 kJ (9.11)
whereas for reaction (9.10) G 298 ≈ 0, but at low temperatures such dispropor-
tionation does not take place.