Page 279 - Handbook of Battery Materials
P. 279
9.3 Metal Hydride–Nickel Batteries 249
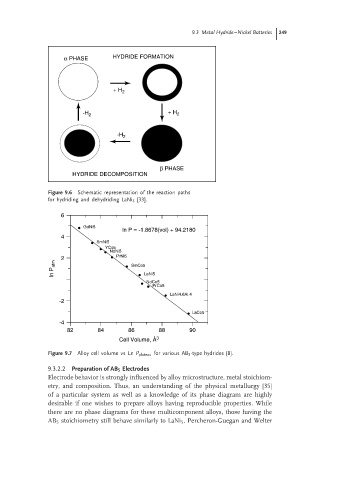
α PHASE HYDRIDE FORMATION
+ H 2
+ H
-H 2 2
-H 2
β PHASE
HYDRIDE DECOMPOSITION
Figure 9.6 Schematic representation of the reaction paths
for hydriding and dehydriding LaNi 5 [33].
6
GdNi5
ln P = -1.8678(vol) + 94.2180
4
SmNi5
YCo5
NdNi5
2 PrNi5
ln P atm SmCo5 LaNi5
NdCo5
PrCo5
LaNi4.6Al.4
-2
LaCo5
-4
82 84 86 88 90
Cell Volume, Å 3
Figure 9.7 Alloy cell volume vs Ln P plateau for various AB 5 -type hydrides [8].
9.3.2.2 Preparation of AB 5 Electrodes
Electrode behavior is strongly influenced by alloy microstructure, metal stoichiom-
etry, and composition. Thus, an understanding of the physical metallurgy [35]
of a particular system as well as a knowledge of its phase diagram are highly
desirable if one wishes to prepare alloys having reproducible properties. While
there are no phase diagrams for these multicomponent alloys, those having the
AB 5 stoichiometry still behave similarly to LaNi 5 . Percheron-Guegan and Welter