Page 283 - Handbook of Battery Materials
P. 283
9.3 Metal Hydride–Nickel Batteries 253
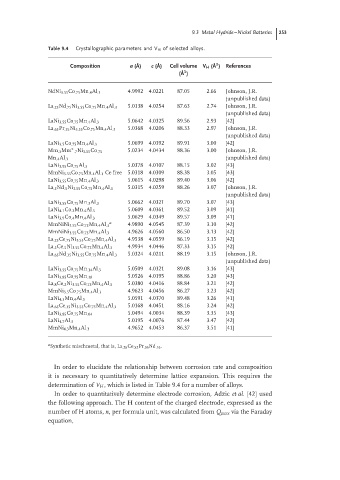
Table 9.4 Crystallographic parameters and V H of selected alloys.
3
Composition a ( ˚ A) c ( ˚ A) Cell volume V H ( ˚ A ) References
3
( ˚ A )
NdNi 3.55 Co .75 Mn .4 Al .3 4.9992 4.0221 87.05 2.66 Johnson, J.R.
(unpublished data)
La .25 Nd .75 Ni 3.55 Co .75 Mn .4 Al .3 5.0138 4.0254 87.63 2.74 Johnson, J.R.
(unpublished data)
5.0642 4.0325 89.56 2.93 [42]
LaNi 3.55 Co .75 Mn .4 Al .3
La .65 Pr .35 Ni 3.55 Co .75 Mn .4 Al .3 5.0368 4.0206 88.33 2.97 Johnson, J.R.
(unpublished data)
LaNi 3.5 Co .75 Mn .4 Al .3 5.0699 4.0392 89.91 3.00 [42]
∗ 5.0234 4.0434 88.36 3.00 Johnson, J.R.
Mm .3 Mm .7 Ni 3.55 Co .75
Mn .4 Al .3 (unpublished data)
LaNi 3.95 Co .75 Al .3 5.0378 4.0107 88.15 3.02 [43]
MmNi 3.55 Co .75 Mn .4 Al .3 Ce free 5.0318 4.0309 88.38 3.05 [43]
LaNi 3.55 Co .75 Mn .4 Al .3 5.0615 4.0298 89.40 3.06 [42]
La .5 Nd .5 Ni 3.55 Co .75 Mn .4 Al .3 5.0315 4.0259 88.26 3.07 Johnson, J.R.
(unpublished data)
LaNi 3.55 Co .75 Mn .3 Al .3 5.0662 4.0321 89.70 3.07 [43]
LaNi 4.1 Co .2 Mn .4 Al .3 5.0609 4.0361 89.52 3.09 [41]
5.0629 4.0349 89.57 3.09 [41]
LaNi 3.9 Co .4 Mn .4 Al .3
a
MmNiNi 3.55 Co .75 Mn .4 Al .3 4.9890 4.0545 87.39 3.10 [42]
MmNiNi 3.55 Co .75 Mn .4 Al .3 4.9626 4.0560 86.50 3.13 [42]
La .25 Ce .75 Ni 3.55 Co .75 Mn .4 Al .3 4.9538 4.0559 86.19 3.15 [42]
La .5 Ce .5 Ni 3.55 Co .75 Mn .4 Al .3 4.9934 4.0446 87.33 3.15 [42]
La .65 Nd .35 Ni 3.55 Co .75 Mn .4 Al .3 5.0324 4.0211 88.19 3.15 Johnson, J.R.
(unpublished data)
LaNi 3.55 Co .75 Mn .14 Al .3 5.0509 4.0321 89.08 3.16 [43]
LaNi 3.85 Co .75 Mn .38 5.0526 4.0195 88.86 3.20 [43]
La .8 Ce .2 Ni 3.55 Co .75 Mn .4 Al .3 5.0380 4.0416 88.84 3.21 [42]
MmNi 3.5 Co .75 Mn .4 Al .3 4.9623 4.0456 86.27 3.23 [42]
LaNi 4.3 Mn .4 Al .3 5.0591 4.0370 89.48 3.26 [41]
La .65 Ce .35 Ni 3.55 Co .75 Mn .4 Al .3 5.0168 4.0451 88.16 3.24 [42]
5.0494 4.0034 88.39 3.35 [43]
LaNi 3.85 Co .75 Mn .04
LaNi 4.7 Al .3 5.0195 4.0076 87.44 3.47 [42]
MmNi 4.3 Mn .4 Al .3 4.9652 4.0453 86.37 3.51 [41]
a Synthetic mischmetal, that is, La .26 Ce .52 Pr .06 Nd .16 .
In order to elucidate the relationship between corrosion rate and composition
it is necessary to quantitatively determine lattice expansion. This requires the
determination of V H , which is listed in Table 9.4 for a number of alloys.
In order to quantitatively determine electrode corrosion, Adzic et al. [42] used
the following approach. The H content of the charged electrode, expressed as the
number of H atoms, n, per formula unit, was calculated from Q max via the Faraday
equation,