Page 287 - Handbook of Battery Materials
P. 287
9.3 Metal Hydride–Nickel Batteries 257
9.3.4.2 Effect of Cobalt
Cobalt is invariably present in commercial MH x battery electrodes. It tends to
increase hydride thermodynamic stability and inhibit corrosion. However, it is
also expensive and substantially increases battery costs; thus, the substitution
of Co by a lower cost metal is desirable. Willems and Buschow [39] attributed
reduced corrosion in LaNi 5−x Co x (x = 1–5) to low V H . Sakai et al. [47] noted that
LaNi 2.5 Co 2.5 was the most durable of a number of substituted LaN 5−x Co x alloys
but it also had the lowest storage capacity.
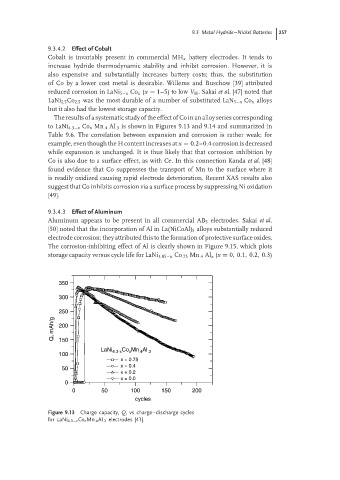
The results of a systematic study of the effect of Co in an alloy series corresponding
to LaNi 4.3−x Co x Mn .4 Al .3 is shown in Figures 9.13 and 9.14 and summarized in
Table 9.6. The correlation between expansion and corrosion is rather weak; for
example, even though the H content increases at x = 0.2–0.4 corrosion is decreased
while expansion is unchanged. It is thus likely that that corrosion inhibition by
Co is also due to a surface effect, as with Ce. In this connection Kanda et al. [48]
found evidence that Co suppresses the transport of Mn to the surface where it
is readily oxidized causing rapid electrode deterioration. Recent XAS results also
suggest that Co inhibits corrosion via a surface process by suppressing Ni oxidation
[49].
9.3.4.3 Effect of Aluminum
Aluminum appears to be present in all commercial AB 5 electrodes. Sakai et al.
[50] noted that the incorporation of Al in La(NiCoAl) 5 alloys substantially reduced
electrode corrosion; they attributed this to the formation of protective surface oxides.
The corrosion-inhibiting effect of Al is clearly shown in Figure 9.15, which plots
storage capacity versus cycle life for LaNi 3.85−x Co .75 Mn .4 Al x (x = 0, 0.1, 0.2, 0.3)
350
300
250
Q, mAh/g 200
150
LaNi 4.3-x Co x Mn .4 Al .3
100
x = 0.75
x = 0.4
50
x = 0.2
x = 0.0
0
0 50 100 150 200
cycles
Figure 9.13 Charge capacity, Q, vs charge–discharge cycles
for LaNi 4.3−x Co x Mn .4 Al .3 electrodes [41].