Page 291 - Handbook of Battery Materials
P. 291
9.5 AB 2 Hydride Electrodes 261
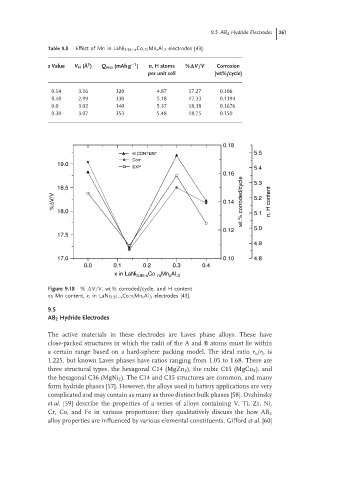
Table 9.8 Effect of Mn in LaNi 3.95−x Co .75 Mn x Al .3 electrodes [43].
−1
3
x Value V H ( ˚ A ) Q max (mAh g ) n,H atoms %∆V/V Corrosion
per unit cell (wt%/cycle)
0.14 3.16 320 4.87 17.27 0.106
0.40 2.99 330 5.18 17.33 0.1394
0.0 3.02 340 5.37 18.38 0.1676
0.30 3.07 353 5.48 18.75 0.150
0.18
H CONTENT 5.5
Corr
19.0
EXP 5.4
0.16
wt.% corroded/cycle n, H content
18.5 5.3
%∆V/V 0.14 5.2
18.0 5.1
0.12 5.0
17.5
4.9
17.0 0.10 4.8
0.0 0.1 0.2 0.3 0.4
x in LaNi 3.95-x Co .75 Mn Al .3
x
Figure 9.18 % V/V, wt % corroded/cycle, and H content
vs Mn content, x,in LaNi 3.95−x Co .75 Mn x Al .3 electrodes [43].
9.5
AB 2 Hydride Electrodes
The active materials in these electrodes are Laves phase alloys. These have
close-packed structures in which the radii of the A and B atoms must lie within
a certain range based on a hard-sphere packing model. The ideal ratio r a /r b is
1.225, but known Laves phases have ratios ranging from 1.05 to 1.68. There are
three structural types, the hexagonal C14 (MgZn 2 ), the cubic C15 (MgCu 2 ), and
the hexagonal C36 (MgNi 2 ). The C14 and C15 structures are common, and many
form hydride phases [57]. However, the alloys used in battery applications are very
complicated and may contain as many as three distinct bulk phases [58]. Ovshinsky
et al. [59] describe the properties of a series of alloys containing V, Ti, Zr, Ni,
Cr, Co, and Fe in various proportions; they qualitatively discuss the how AB 2
alloy properties are influenced by various elemental constituents. Gifford et al. [60]