Page 290 - Handbook of Battery Materials
P. 290
260 9 Metal Hydride Electrodes
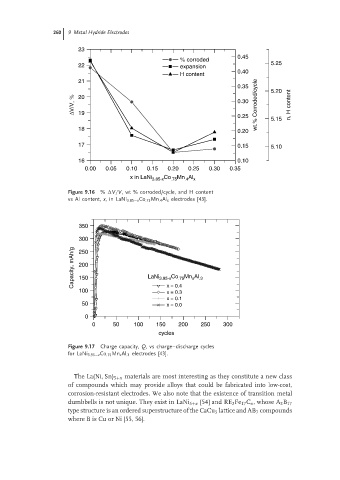
23
0.45
% corroded
22 expansion 5.25
0.40
H content
21
0.35 5.20
∆V/V, % 20 0.30 wt.% Corroded/cycle n, H content
19
0.25 5.15
18 0.20
17 0.15 5.10
16 0.10
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35
x in LaNi 3.85-x Co .75 Mn Al x
.4
Figure 9.16 % V/V, wt % corroded/cycle, and H content
vs Al content, x,in LaNi 3.85−x Co .75 Mn .4 Al x electrodes [43].
350
300
Capacity, mAh/g 200 LaNi 3.95-x Co .75 Mn x Al .3
250
150
100 x = 0.4
x = 0.3
x = 0.1
50 x = 0.0
0
0 50 100 150 200 250 300
cycles
Figure 9.17 Charge capacity, Q, vs charge–discharge cycles
for LaNi 3.95−x Co .75 Mn x Al .3 electrodes [43].
The La(Ni, Sn) 5+x materials are most interesting as they constitute a new class
of compounds which may provide alloys that could be fabricated into low-cost,
corrosion-resistant electrodes. We also note that the existence of transition metal
dumbbells is not unique. They exist in LaNi 5+x [54] and RE 2 Fe 17 C x , whose A 2 B 17
type structure is an ordered superstructure of the CaCu 5 lattice and AB 7 compounds
where B is Cu or Ni [55, 56].