Page 294 - Handbook of Battery Materials
P. 294
264 9 Metal Hydride Electrodes
300
250
200
Q, mAh 150
Zr 1-x Ti V Ni 1.1 Fe Mn .2
x .5
.2
100
x = 0.75
x = 0.5
50 x = 0.25
x = 0.0
0
0 50 100 150 200 250 300
Cycles
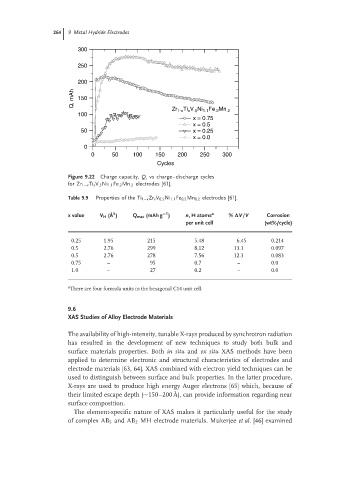
Figure 9.22 Charge capacity, Q, vs charge–discharge cycles
for Zr 1−x Ti x V .5 Ni 1.1 Fe .2 Mn .2 electrodes [61].
Table 9.9 Properties of the Ti 1−x Zr x V 0.5 Ni 1.1 Fe 0.2 Mn 0.2 electrodes [61].
3
−1
xvalue V H ( ˚ A ) Q max (mAh g ) n,H atoms a % ∆V/V Corrosion
per unit cell (wt%/cycle)
0.25 1.95 215 5.48 6.45 0.214
0.5 2.76 299 8.12 13.1 0.097
0.5 2.76 278 7.56 12.3 0.083
0.75 – 95 0.7 – 0.0
1.0 – 27 0.2 – 0.0
a
There are four formula units in the hexagonal C14 unit cell.
9.6
XAS Studies of Alloy Electrode Materials
The availability of high-intensity, tunable X-rays produced by synchrotron radiation
has resulted in the development of new techniques to study both bulk and
surface materials properties. Both in situ and ex situ XAS methods have been
applied to determine electronic and structural characteristics of electrodes and
electrode materials [63, 64]. XAS combined with electron yield techniques can be
used to distinguish between surface and bulk properties. In the latter procedure,
X-rays are used to produce high energy Auger electrons [65] which, because of
their limited escape depth (∼150–200 ˚ A), can provide information regarding near
surface composition.
The element-specific nature of XAS makes it particularly useful for the study
of complex AB 5 and AB 2 MH electrode materials. Mukerjee et al. [46] examined