Page 289 - Handbook of Battery Materials
P. 289
9.4 Super-Stoichiometric AB 5+x Alloys 259
400
350 LaNi 3.85-x Co .75 Mn .4 Al x
300
Capacity, Q, mAh/g 200 x = 0.3
250
150
x = 0.2
100
50 x = 0.1
x = 0.0
0
-50
0 25 50 75 100 125 150
Cycles
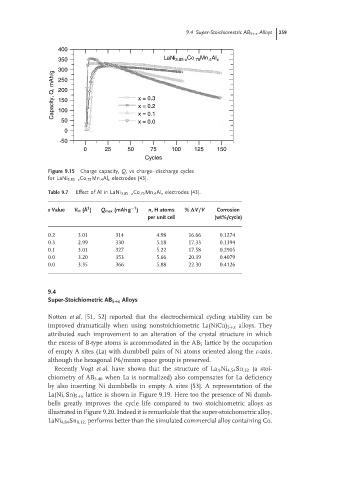
Figure 9.15 Charge capacity, Q, vs charge–discharge cycles
for LaNi 3.85−x Co .75 Mn .4 Al x electrodes [43].
Table 9.7 Effect of Al in LaNi 3.85−x Co .75 Mn .4 Al x electrodes [43].
3
−1
x Value V H ( ˚ A ) Q max (mAh g ) n,H atoms % ∆V/V Corrosion
per unit cell (wt%/cycle)
0.2 3.01 314 4.98 16.66 0.1274
0.3 2.99 330 5.18 17.33 0.1394
0.1 3.01 327 5.22 17.58 0.2905
0.0 3.20 353 5.66 20.39 0.4079
0.0 3.35 366 5.88 22.30 0.4126
9.4
Super-Stoichiometric AB 5+x Alloys
Notten et al. [51, 52] reported that the electrochemical cycling stability can be
improved dramatically when using nonstoichiometric La(NiCu) 5+x alloys. They
attributed such improvement to an alteration of the crystal structure in which
the excess of B-type atoms is accommodated in the AB 5 lattice by the occupation
of empty A sites (La) with dumbbell pairs of Ni atoms oriented along the c-axis,
although the hexagonal P6/mmm space group is preserved.
Recently Vogt et al. have shown that the structure of La .9 Ni 4.54 Sn .32 (a stoi-
chiometry of AB 5.40 when La is normalized) also compensates for La deficiency
by also inserting Ni dumbbells in empty A sites [53]. A representation of the
La(Ni, Sn) 5+x lattice is shown in Figure 9.19. Here too the presence of Ni dumb-
bells greatly improves the cycle life compared to two stoichiometric alloys as
illustrated in Figure 9.20. Indeed it is remarkable that the super-stoichometric alloy,
LaNi 4.84 Sn 0.32, performs better than the simulated commercial alloy containing Co.