Page 288 - Handbook of Battery Materials
P. 288
258 9 Metal Hydride Electrodes
18.6
Corrosion
0.5
expansion 5.25
18.4 H content
0.4
18.2 5.20
∆V/V, % wt. % Corroded/cycle n, H content
18.0 0.3 5.15
0.2
17.8
5.10
17.6 0.1
0.0 0.2 0.4 0.6 0.8
x in LaNi 4.3-x Co x Mn .4 Al .3
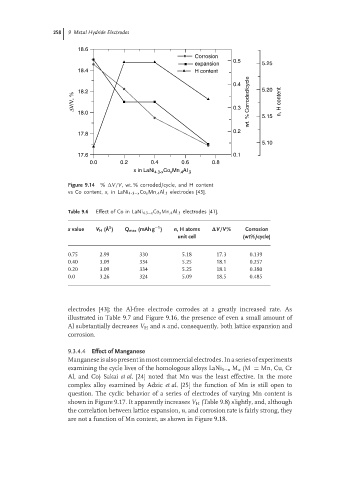
Figure 9.14 % V/V, wt. % corroded/cycle, and H content
vs Co content, x,in LaNi 4 . 3−x Co x Mn .4 Al .3 electrodes [43].
Table 9.6 Effect of Co in LaNi 4.3−x Co x Mn .4 Al .3 electrodes [41].
3
−1
x value V H ( ˚ A ) Q max (mAh g ) n, H atoms ∆V/V% Corrosion
unit cell (wt%/cycle)
0.75 2.99 330 5.18 17.3 0.139
0.40 3.09 334 5.25 18.1 0.257
0.20 3.09 334 5.25 18.1 0.380
0.0 3.26 324 5.09 18.5 0.485
electrodes [43]; the Al-free electrode corrodes at a greatly increased rate. As
illustrated in Table 9.7 and Figure 9.16, the presence of even a small amount of
Al substantially decreases V H and n and, consequently, both lattice expansion and
corrosion.
9.3.4.4 Effect of Manganese
Manganese is also present in most commercial electrodes. In a series of experiments
examining the cycle lives of the homologous alloys LaNi 5−x M x (M = Mn, Cu, Cr
Al, and Co) Sakai et al. [24] noted that Mn was the least effective. In the more
complex alloy examined by Adzic et al. [25] the function of Mn is still open to
question. The cyclic behavior of a series of electrodes of varying Mn content is
shown in Figure 9.17. It apparently increases V H (Table 9.8) slightly, and, although
the correlation between lattice expansion, n, and corrosion rate is fairly strong, they
are not a function of Mn content, as shown in Figure 9.18.