Page 73 - Handbook of Natural Gas Transmission and Processing Principles and Practices
P. 73
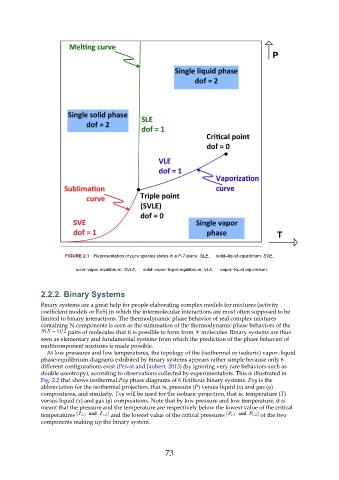
FIGURE 2.1 Representation of pure species states in a P-T plane. SLE, solid–liquid equilibrium; SVE,
solid–vapor equilibrium; SVLE, solid–vapor–liquid equilibrium; VLE, vapor–liquid equilibrium.
2.2.2. Binary Systems
Binary systems are a great help for people elaborating complex models for mixtures (activity
coefficient models or EoS) in which the intermolecular interactions are most often supposed to be
limited to binary interactions. The thermodynamic phase behavior of real complex mixtures
containing N components is seen as the summation of the thermodynamic phase behaviors of the
pairs of molecules that it is possible to form from molecules. Binary systems are thus
seen as elementary and fundamental systems from which the prediction of the phase behavior of
multicomponent mixtures is made possible.
At low pressures and low temperatures, the topology of the (isothermal or isobaric) vapor–liquid
phase-equilibrium diagrams exhibited by binary systems appears rather simple because only 6
different configurations exist (Privat and Jaubert, 2013) (by ignoring very rare behaviors such as
double azeotropy), according to observations collected by experimentalists. This is illustrated in
Fig. 2.2 that shows isothermal Pxy phase diagrams of 6 fictitious binary systems. Pxy is the
abbreviation for the isothermal projection, that is, pressure (P) versus liquid (x) and gas (y)
compositions, and similarly, Txy will be used for the isobaric projection, that is, temperature (T)
versus liquid (x) and gas (y) compositions. Note that by low pressure and low temperature, it is
meant that the pressure and the temperature are respectively below the lowest value of the critical
temperatures and the lowest value of the critical pressures of the two
components making up the binary system.
73