Page 76 - Handbook of Natural Gas Transmission and Processing Principles and Practices
P. 76
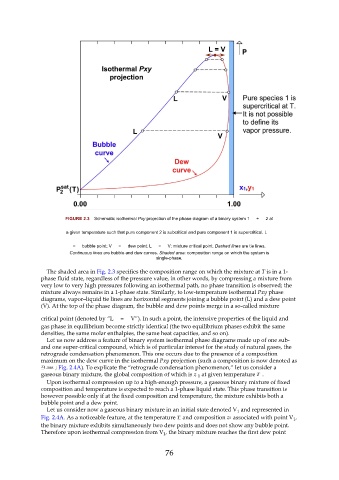
FIGURE 2.3 Schematic isothermal Pxy projection of the phase diagram of a binary system 1 + 2 at
a given temperature such that pure component 2 is subcritical and pure component 1 is supercritical. L
= bubble point; V = dew point; L = V: mixture critical point. Dashed lines are tie lines.
Continuous lines are bubble and dew curves. Shaded area: composition range on which the system is
single-phase.
The shaded area in Fig. 2.3 specifies the composition range on which the mixture at T is in a 1-
phase fluid state, regardless of the pressure value, in other words, by compressing a mixture from
very low to very high pressures following an isothermal path, no phase transition is observed; the
mixture always remains in a 1-phase state. Similarly, to low-temperature isothermal Pxy phase
diagrams, vapor–liquid tie lines are horizontal segments joining a bubble point (L) and a dew point
(V). At the top of the phase diagram, the bubble and dew points merge in a so-called mixture
critical point (denoted by “L = V”). In such a point, the intensive properties of the liquid and
gas phase in equilibrium become strictly identical (the two equilibrium phases exhibit the same
densities, the same molar enthalpies, the same heat capacities, and so on).
Let us now address a feature of binary system isothermal phase diagrams made up of one sub-
and one super-critical compound, which is of particular interest for the study of natural gases, the
retrograde condensation phenomenon. This one occurs due to the presence of a composition
maximum on the dew curve in the isothermal Pxy projection (such a composition is now denoted as
; Fig. 2.4A). To explicate the “retrograde condensation phenomenon,” let us consider a
gaseous binary mixture, the global composition of which is z at given temperature .
1
Upon isothermal compression up to a high-enough pressure, a gaseous binary mixture of fixed
composition and temperature is expected to reach a 1-phase liquid state. This phase transition is
however possible only if at the fixed composition and temperature, the mixture exhibits both a
bubble point and a dew point.
Let us consider now a gaseous binary mixture in an initial state denoted V and represented in
1
Fig. 2.4A. As a noticeable feature, at the temperature and composition associated with point V ,
1
the binary mixture exhibits simultaneously two dew points and does not show any bubble point.
Therefore upon isothermal compression from V , the binary mixture reaches the first dew point
1
76