Page 81 - Handbook of Natural Gas Transmission and Processing Principles and Practices
P. 81
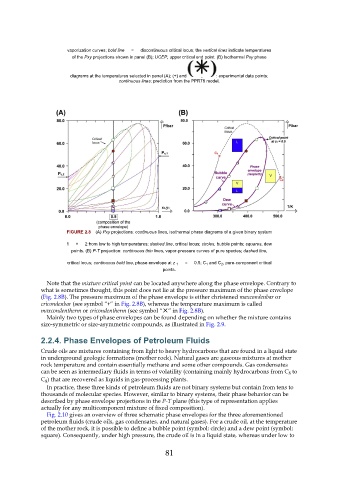
vaporization curves; bold line = discontinuous critical locus; the vertical lines indicate temperatures
of the Pxy projections shown in panel (B); UCEP, upper critical end point. (B) Isothermal Pxy phase
diagrams at the temperatures selected in panel (A); (+) and : experimental data points;
continuous lines: prediction from the PPR78 model.
FIGURE 2.8 (A) Pxy projections: continuous lines, isothermal phase diagrams of a given binary system
1 + 2 from low to high temperatures; dashed line, critical locus; circles, bubble points; squares, dew
points. (B) P-T projection: continuous thin lines, vapor-pressure curves of pure species; dashed line,
critical locus; continuous bold line, phase envelope at z 1 = 0.5; C 1 and C 2 , pure-component critical
points.
Note that the mixture critical point can be located anywhere along the phase envelope. Contrary to
what is sometimes thought, this point does not lie at the pressure maximum of the phase envelope
(Fig. 2.8B). The pressure maximum of the phase envelope is either christened maxcondenbar or
cricondenbar (see symbol “+” in Fig. 2.8B), whereas the temperature maximum is called
maxcondentherm or cricondentherm (see symbol “✕” in Fig. 2.8B).
Mainly two types of phase envelopes can be found depending on whether the mixture contains
size-symmetric or size-asymmetric compounds, as illustrated in Fig. 2.9.
2.2.4. Phase Envelopes of Petroleum Fluids
Crude oils are mixtures containing from light to heavy hydrocarbons that are found in a liquid state
in underground geologic formations (mother rock). Natural gases are gaseous mixtures at mother
rock temperature and contain essentially methane and some other compounds. Gas condensates
can be seen as intermediary fluids in terms of volatility (containing mainly hydrocarbons from C to
5
C ) that are recovered as liquids in gas-processing plants.
8
In practice, these three kinds of petroleum fluids are not binary systems but contain from tens to
thousands of molecular species. However, similar to binary systems, their phase behavior can be
described by phase envelope projections in the P-T plane (this type of representation applies
actually for any multicomponent mixture of fixed composition).
Fig. 2.10 gives an overview of three schematic phase envelopes for the three aforementioned
petroleum fluids (crude oils, gas condensates, and natural gases). For a crude oil, at the temperature
of the mother rock, it is possible to define a bubble point (symbol: circle) and a dew point (symbol:
square). Consequently, under high pressure, the crude oil is in a liquid state, whereas under low to
81