Page 191 - Handbook of Plastics Technologies
P. 191
THERMOSETS
THERMOSETS 3.61
of properties. The preferred polyisocyanates are polymeric methylene diphenyl isocyan-
ates with 2 to 7 isocyanate groups per molecule. Mechanical properties can be improved
by glass fiber reinforcement. Copolymerization with epoxy resins to form polyoxazoli-
dones has also been suggested (Fig. 3.65).
FIGURE 3.65 Copolymerization of isocyanate and epoxy to
polyoxazolidone.
3.1.7.2.3 Hexaazatriphenylene Trianhydride. Researchers seeking to take polyim-
ides to their maximum temperature limits have often concluded that the remaining weak
points in their structure are the H atoms. Using hexaazatriphenylene trianhydride
(Fig. 3.66) to form hydrogen-free polyimides, they have been able to produced polyimides
good up to 700°C in air.
FIGURE 3.66 Hexaazatriphenylene trianhy-
dride.
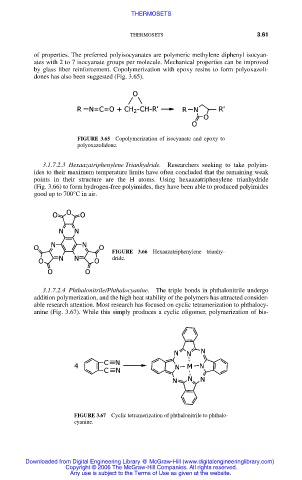
3.1.7.2.4 Phthalonitrile/Phthalocyanine. The triple bonds in phthalonitrile undergo
addition polymerization, and the high heat stability of the polymers has attracted consider-
able research attention. Most research has focused on cyclic tetramerization to phthalocy-
anine (Fig. 3.67). While this simply produces a cyclic oligomer, polymerization of bis-
FIGURE 3.67 Cyclic tetramerization of phthalonitrile to phthalo-
cyanine.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.