Page 242 - Handbook of Plastics Technologies
P. 242
ELASTOMERS
4.34 CHAPTER 4
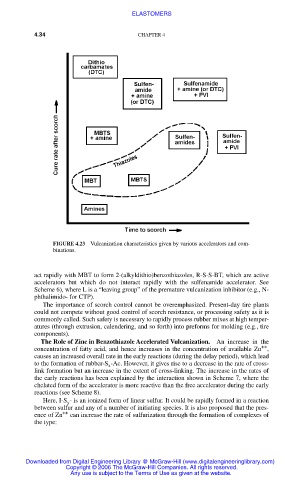
FIGURE 4.23 Vulcanization characteristics given by various accelerators and com-
binations.
act rapidly with MBT to form 2-(alkyldithio)benzothiazoles, R-S-S-BT, which are active
accelerators but which do not interact rapidly with the sulfenamide accelerator. See
Scheme 6), where L is a “leaving group” of the premature vulcanization inhibitor (e.g., N-
phthalimido- for CTP).
The importance of scorch control cannot be overemphasized. Present-day tire plants
could not compete without good control of scorch resistance, or processing safety as it is
commonly called. Such safety is necessary to rapidly process rubber mixes at high temper-
atures (through extrusion, calendering, and so forth) into preforms for molding (e.g., tire
components).
The Role of Zinc in Benzothiazole Accelerated Vulcanization. An increase in the
++
concentration of fatty acid, and hence increases in the concentration of available Zn ,
causes an increased overall rate in the early reactions (during the delay period), which lead
to the formation of rubber-S -Ac. However, it gives rise to a decrease in the rate of cross-
x
link formation but an increase in the extent of cross-linking. The increase in the rates of
the early reactions has been explained by the interaction shown in Scheme 7, where the
chelated form of the accelerator is more reactive than the free accelerator during the early
reactions (see Scheme 8).
Here, I-S - is an ionized form of linear sulfur. It could be rapidly formed in a reaction
y
between sulfur and any of a number of initiating species. It is also proposed that the pres-
++
ence of Zn can increase the rate of sulfurization through the formation of complexes of
the type:
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.