Page 241 - Handbook of Plastics Technologies
P. 241
ELASTOMERS
ELASTOMERS 4.33
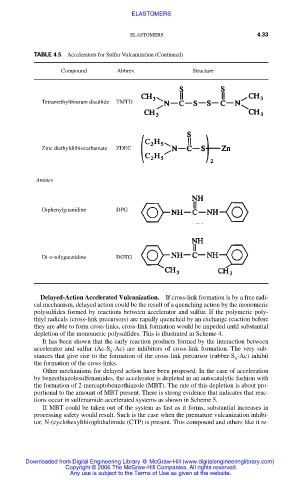
TABLE 4.5 Accelerators for Sulfur Vulcanization (Continued)
Compound Abbrev. Structure
Tetramethylthiuram disulfide TMTD
Zinc diethyldithiocarbamate ZDEC
Amines
Diphenylguanidine DPG
Di-o-tolyguanidine DOTG
Delayed-Action Accelerated Vulcanization. If cross-link formation is by a free radi-
cal mechanism, delayed action could be the result of a quenching action by the monomeric
polysulfides formed by reactions between accelerator and sulfur. If the polymeric poly-
thiyl radicals (cross-link precursors) are rapidly quenched by an exchange reaction before
they are able to form cross-links, cross-link formation would be impeded until substantial
depletion of the monomeric polysulfides. This is illustrated in Scheme 4.
It has been shown that the early reaction products formed by the interaction between
accelerator and sulfur (Ac-S -Ac) are inhibitors of cross-link formation. The very sub-
x
stances that give rise to the formation of the cross-link precursor (rubber-S -Ac) inhibit
x
the formation of the cross-links.
Other mechanisms for delayed action have been proposed. In the case of acceleration
by benzothiazolesulfenamides, the accelerator is depleted in an autocatalytic fashion with
the formation of 2-mercaptobenzothiazole (MBT). The rate of this depletion is about pro-
portional to the amount of MBT present. There is strong evidence that indicates that reac-
tions occur in sulfenamide-accelerated systems as shown in Scheme 5.
If MBT could be taken out of the system as fast as it forms, substantial increases in
processing safety would result. Such is the case when the premature vulcanization inhibi-
tor, N-(cyclohexylthio)phthalimide (CTP) is present. This compound and others like it re-
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.