Page 236 - Handbook of Plastics Technologies
P. 236
ELASTOMERS
4.28 CHAPTER 4
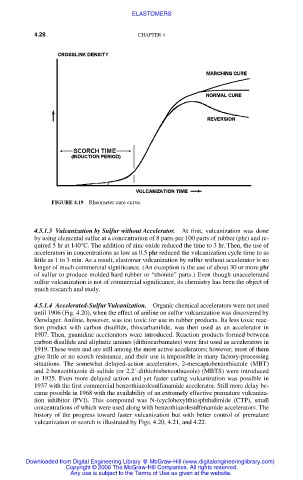
FIGURE 4.19 Rheometer cure curve.
4.5.1.3 Vulcanization by Sulfur without Accelerator. At first, vulcanization was done
by using elemental sulfur at a concentration of 8 parts per 100 parts of rubber (phr) and re-
quired 5 hr at 140°C. The addition of zinc oxide reduced the time to 3 hr. Then, the use of
accelerators in concentrations as low as 0.5 phr reduced the vulcanization cycle time to as
little as 1 to 3 min. As a result, elastomer vulcanization by sulfur without accelerator is no
longer of much commercial significance. (An exception is the use of about 30 or more phr
of sulfur to produce molded hard rubber or “ebonite” parts.) Even though unaccelerated
sulfur vulcanization is not of commercial significance, its chemistry has been the object of
much research and study.
4.5.1.4 Accelerated-Sulfur Vulcanization. Organic chemical accelerators were not used
until 1906 (Fig. 4.20), when the effect of aniline on sulfur vulcanization was discovered by
Oenslager. Aniline, however, was too toxic for use in rubber products. Its less toxic reac-
tion product with carbon disulfide, thiocarbanilide, was then used as an accelerator in
1907. Then, guanidine accelerators were introduced. Reaction products formed between
carbon disulfide and aliphatic amines (dithiocarbamates) were first used as accelerators in
1919. These were and are still among the most active accelerators; however, most of them
give little or no scorch resistance, and their use is impossible in many factory-processing
situations. The somewhat delayed-action accelerators, 2-mercaptobenzothiazole (MBT)
and 2-benzothiazole di-sulfide (or 2,2´-dithiobisbenzothiazole) (MBTS) were introduced
in 1925. Even more delayed action and yet faster curing vulcanization was possible in
1937 with the first commercial benzothiazolesulfenamide accelerator. Still more delay be-
came possible in 1968 with the availability of an extremely effective premature vulcaniza-
tion inhibitor (PVI). This compound was N-(cyclohexylthio)phthalimide (CTP), small
concentrations of which were used along with benzothiazolesulfenamide accelerators. The
history of the progress toward faster vulcanization but with better control of premature
vulcanization or scorch is illustrated by Figs. 4.20, 4.21, and 4.22.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.