Page 239 - Handbook of Plastics Technologies
P. 239
ELASTOMERS
ELASTOMERS 4.31
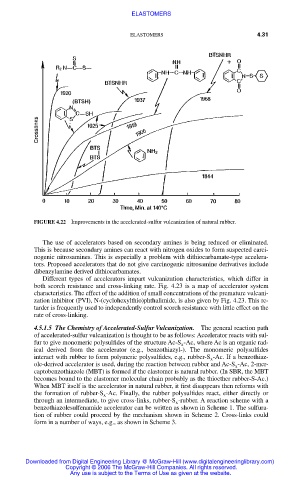
FIGURE 4.22 Improvements in the accelerated-sulfur vulcanization of natural rubber.
The use of accelerators based on secondary amines is being reduced or eliminated.
This is because secondary amines can react with nitrogen oxides to form suspected carci-
nogenic nitrosamines. This is especially a problem with dithiocarbamate-type accelera-
tors. Proposed accelerators that do not give carcinogenic nitrosamine derivatives include
dibenzylamine derived dithiocarbamates.
Different types of accelerators impart vulcanization characteristics, which differ in
both scorch resistance and cross-linking rate. Fig. 4.23 is a map of accelerator system
characteristics. The effect of the addition of small concentrations of the premature vulcani-
zation inhibitor (PVI), N-(cyclohexylthio)phthalimide, is also given by Fig. 4.23. This re-
tarder is frequently used to independently control scorch resistance with little effect on the
rate of cross-linking.
4.5.1.5 The Chemistry of Accelerated-Sulfur Vulcanization. The general reaction path
of accelerated-sulfur vulcanization is thought to be as follows: Accelerator reacts with sul-
fur to give monomeric polysulfides of the structure Ac-S -Ac, where Ac is an organic rad-
x
ical derived from the accelerator (e.g., benzothiazyl-). The monomeric polysulfides
interact with rubber to form polymeric polysulfides, e.g., rubber-S -Ac. If a benzothiaz-
x
ole-derived accelerator is used, during the reaction between rubber and Ac-S -Ac, 2-mer-
x
captobenzothiazole (MBT) is formed if the elastomer is natural rubber. (In SBR, the MBT
becomes bound to the elastomer molecular chain probably as the thioether rubber-S-Ac.)
When MBT itself is the accelerator in natural rubber, it first disappears then reforms with
the formation of rubber-S -Ac. Finally, the rubber polysulfides react, either directly or
x
through an intermediate, to give cross-links, rubber-S -rubber. A reaction scheme with a
x
benzothiazolesulfenamide accelerator can be written as shown in Scheme 1. The sulfura-
tion of rubber could proceed by the mechanism shown in Scheme 2. Cross-links could
form in a number of ways, e.g., as shown in Scheme 3.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.