Page 244 - Handbook of Plastics Technologies
P. 244
ELASTOMERS
4.36 CHAPTER 4
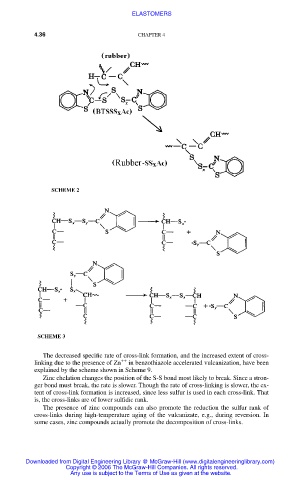
SCHEME 2
SCHEME 3
The decreased specific rate of cross-link formation, and the increased extent of cross-
++
linking due to the presence of Zn in benzothiazole accelerated vulcanization, have been
explained by the scheme shown in Scheme 9.
Zinc chelation changes the position of the S-S bond most likely to break. Since a stron-
ger bond must break, the rate is slower. Though the rate of cross-linking is slower, the ex-
tent of cross-link formation is increased, since less sulfur is used in each cross-link. That
is, the cross-links are of lower sulfidic rank.
The presence of zinc compounds can also promote the reduction the sulfur rank of
cross-links during high-temperature aging of the vulcanizate, e.g., during reversion. In
some cases, zinc compounds actually promote the decomposition of cross-links.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.