Page 249 - Handbook of Plastics Technologies
P. 249
ELASTOMERS
ELASTOMERS 4.41
hardening (the so-called marching modulus) during extensive overcure. In natural rubber
and synthetic isoprene-polymer rubbers, the cross-links tend to be more polysulfidic than
in the case of BR or SBR. The highly polysulfidic cross-links are more heat-labile than
their lower rank cousins in BR and SBR; they are more likely to break and then form cy-
clic chain modifications.
The effect of zinc is much greater in the vulcanization of isoprene rubbers than it is in
the vulcanization of BR and SBR. Again, the reason for the difference is not known, but a
strong speculation is that this difference is also related to the presence of methyl groups
only in the case of the isoprene rubbers.
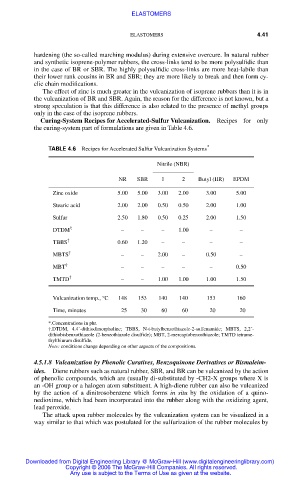
Curing-System Recipes for Accelerated-Sulfur Vulcanization. Recipes for only
the curing-system part of formulations are given in Table 4.6.
TABLE 4.6 Recipes for Accelerated Sulfur Vulcanization Systems *
Nitrile (NBR)
NR SBR 1 2 Butyl (IIR) EPDM
Zinc oxide 5.00 5.00 3.00 2.00 3.00 5.00
Stearic acid 2.00 2.00 0.50 0.50 2.00 1.00
Sulfur 2.50 1.80 0.50 0.25 2.00 1.50
DTDM † – – – 1.00 – –
TBBS † 0.60 1.20 – – – –
MBTS † – – 2.00 – 0.50 –
MBT † – – – – – 0.50
TMTD † – – 1.00 1.00 1.00 1.50
Vulcanization temp., °C 148 153 140 140 153 160
Time, minutes 25 30 60 60 20 20
*.Concentrations in phr.
†.DTDM, 4.4´-dithiodimorpholine; TBBS, N-t-butylbenzothiazole-2-sulfenamide; MBTS, 2,2´-
dithiobisbenzothiazole (2-benzothiazole disulfide); MBT, 2-mercaptobenzothiazole; TMTD tetrame-
thylthiuram disulfide.
Note: conditions change depending on other aspects of the compositions.
4.5.1.8 Vulcanization by Phenolic Curatives, Benzoquinone Derivatives or Bismaleim-
ides. Diene rubbers such as natural rubber, SBR, and BR can be vulcanized by the action
of phenolic compounds, which are (usually di-substituted by -CH2-X groups where X is
an -OH group or a halogen atom substituent. A high-diene rubber can also be vulcanized
by the action of a dinitrosobenzene which forms in situ by the oxidation of a quino-
nedioxime, which had been incorporated into the rubber along with the oxidizing agent,
lead peroxide.
The attack upon rubber molecules by the vulcanization system can be visualized in a
way similar to that which was postulated for the sulfurization of the rubber molecules by
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.