Page 257 - Handbook of Plastics Technologies
P. 257
ELASTOMERS
ELASTOMERS 4.49
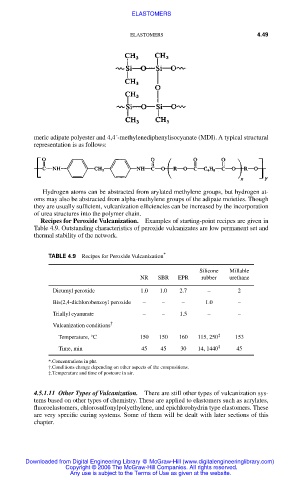
meric adipate polyester and 4,4´-methylenediphenylisocyanate (MDI). A typical structural
representation is as follows:
Hydrogen atoms can be abstracted from arylated methylene groups, but hydrogen at-
oms may also be abstracted from alpha-methylene groups of the adipate moieties. Though
they are usually sufficient, vulcanization efficiencies can be increased by the incorporation
of urea structures into the polymer chain.
Recipes for Peroxide Vulcanization. Examples of starting-point recipes are given in
Table 4.9. Outstanding characteristics of peroxide vulcanizates are low permanent set and
thermal stability of the network.
TABLE 4.9 Recipes for Peroxide Vulcanization *
Silicone Millable
NR SBR EPR rubber urethane
Dicumyl peroxide 1.0 1.0 2.7 – 2
Bis(2,4-dichlorobenzoyl peroxide – – – 1.0 –
Triallyl cyanurate – – 1.5 – –
Vulcanization conditions †
Temperature, °C 150 150 160 115, 250 ‡ 153
Time, min 45 45 30 14, 1440 ‡ 45
*.Concentrations in phr.
†.Conditions change depending on other aspects of the compositions.
‡.Temperature and time of postcure in air.
4.5.1.11 Other Types of Vulcanization. There are still other types of vulcanization sys-
tems based on other types of chemistry. These are applied to elastomers such as acrylates,
fluoroelastomers, chlorosulfonylpolyethylene, and epichlorohydrin type elastomers. These
are very specific curing systems. Some of them will be dealt with later sections of this
chapter.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.