Page 259 - Handbook of Plastics Technologies
P. 259
ELASTOMERS
ELASTOMERS 4.51
Different radical types exist in different diene polymers; e.g., polyisoprene produces
tertiary alkyl radicals, polybutadiene gives secondary alkyl types, and SBR gives allylic
and benzylic types, while a nitrile polymer gives allylic and tertiary cyano types. These
radicals and their corresponding peroxy radicals interact with parts of their own polymer
chains, giving shielding effects, rearrangements, cleavages, and internal additions to
neighboring double bonds. Also, free radicals undergo disproportionation, addition to car-
bon-carbon double bonds, and coupling.
Polyisoprene softens on oxidative aging, while rubber polymers, which are based on
butadiene, harden on aging. The tertiary allylic radical in polyisoprene, being stericly pro-
tected, does not easily undergo cross-linking with other radicals or neighboring chain dou-
ble bonds. It reacts primarily with oxygen and subsequently leads to polymer cleavage.
The secondary allylic system is more reactive. It can undergo cross-chain reactions by rad-
ical addition; this can cause hardening of the composition.
4.5.2.2 Antioxidants. Factors affecting the performance of antioxidants include the in-
trinsic activity of the antioxidant system, the solubility of the antioxidant system in the
polymer matrix, and the volatility of the antioxidant. For rubber, unfortunately, the most
effective antioxidants are the staining and discoloring derivatives of aryl amines; however,
the need for nondiscoloring antioxidants has been filled by phenolic nonstaining antioxi-
dants. Amines find application as both raw polymer stabilizers and also as final vulcani-
zate stabilizers. On the other hand, phenolics can be used in nonblack reinforced
vulcanizates where nonstaining and nondiscoloration is desired.
Amine and phenolic antioxidants are considered free-radical scavengers. They proba-
bly work by the direct abstraction of amine hydrogen by the RO• group. Increased steric
hindrance at the 2 and 6 position of phenolic antioxidant has resulted in improved antioxi-
dant performance. There may be an optimum amount of steric hindrance of the phenolic
group, which should be matched to the oxidizable matrix polymer. This will allow a bal-
anced interference of the radical chain process so that both propagating species, R• and
RO•, are effectively neutralized.
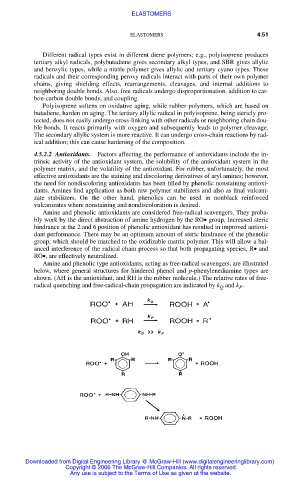
Amine and phenolic type antioxidants, acting as free-radical scavengers, are illustrated
below, where general structures for hindered phenol and p-phenylenediamine types are
shown. (AH is the antioxidant, and RH is the rubber molecule.) The relative rates of free-
radical quenching and free-radical-chain propagation are indicated by k and k .
Q
P
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.