Page 79 - Handbook of Plastics Technologies
P. 79
THERMOPLASTICS
THERMOPLASTICS 2.19
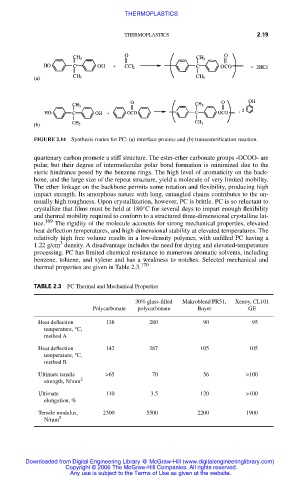
FIGURE 2.14 Synthesis routes for PC: (a) interface process and (b) transesterification reaction.
quartenary carbon promote a stiff structure. The ester-ether carbonate groups -OCOO- are
polar, but their degree of intermolecular polar bond formation is minimized due to the
steric hindrance posed by the benzene rings. The high level of aromaticity on the back-
bone, and the large size of the repeat structure, yield a molecule of very limited mobility.
The ether linkage on the backbone permits some rotation and flexibility, producing high
impact strength. Its amorphous nature with long, entangled chains contributes to the un-
usually high toughness. Upon crystallization, however, PC is brittle. PC is so reluctant to
crystallize that films must be held at 180°C for several days to impart enough flexibility
and thermal mobility required to conform to a structured three-dimensional crystalline lat-
tice. 169 The rigidity of the molecule accounts for strong mechanical properties, elevated
heat deflection temperatures, and high dimensional stability at elevated temperatures. The
relatively high free volume results in a low-density polymer, with unfilled PC having a
3
1.22 g/cm density. A disadvantage includes the need for drying and elevated-temperature
processing. PC has limited chemical resistance to numerous aromatic solvents, including
benzene, toluene, and xylene and has a weakness to notches. Selected mechanical and
170
thermal properties are given in Table 2.3.
TABLE 2.3 PC Thermal and Mechanical Properties
30% glass-filled Makroblend PR51, Xenoy, CL101
Polycarbonate polycarbonate Bayer GE
Heat deflection 138 280 90 95
temperature, °C,
method A
Heat deflection 142 287 105 105
temperature, °C,
method B
Ultimate tensile >65 70 56 >100
strength, N/mm 2
Ultimate 110 3.5 120 >100
elongation, %
Tensile modulus, 2300 5500 2200 1900
N/mm 2
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.