Page 91 - Handbook of Plastics Technologies
P. 91
THERMOPLASTICS
THERMOPLASTICS 2.31
parts, are used in extrusion coating, coextrusions, and laminating applications as heat-seal
layers. EMA is one of the most thermally stable of this group, and as such it is commonly
used to form heat and RF seals as well in multiextrusion tie-layer applications. This copol-
ymer is also widely used as a blending compound with olefin homopolymers (VLDPE,
LLDPE, LDPE, and PP) as well as with polyamides, polyesters, and polycarbonate to im-
prove impact strength and toughness and to increase either heat seal response or to pro-
232
mote adhesion. EMA is also used in soft blow-molded articles such as squeeze toys,
tubing, disposable medical gloves, and foamed sheet. EMA copolymers and EEA copoly-
mers containing up to 8 percent ethyl acrylate are approved by the FDA for food packag-
233
ing.
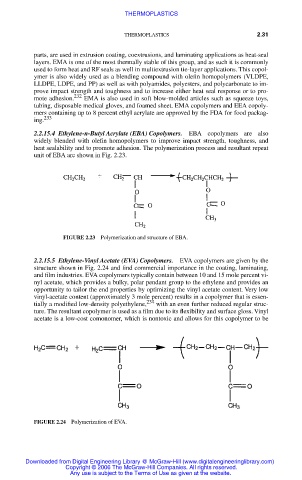
2.2.15.4 Ethylene-n-Butyl Acrylate (EBA) Copolymers. EBA copolymers are also
widely blended with olefin homopolymers to improve impact strength, toughness, and
heat sealability and to promote adhesion. The polymerization process and resultant repeat
unit of EBA are shown in Fig. 2.23.
FIGURE 2.23 Polymerization and structure of EBA.
2.2.15.5 Ethylene-Vinyl Acetate (EVA) Copolymers. EVA copolymers are given by the
structure shown in Fig. 2.24 and find commercial importance in the coating, laminating,
and film industries. EVA copolymers typically contain between 10 and 15 mole percent vi-
nyl acetate, which provides a bulky, polar pendant group to the ethylene and provides an
opportunity to tailor the end properties by optimizing the vinyl acetate content. Very low
vinyl-acetate content (approximately 3 mole percent) results in a copolymer that is essen-
234
tially a modified low-density polyethylene, with an even further reduced regular struc-
ture. The resultant copolymer is used as a film due to its flexibility and surface gloss. Vinyl
acetate is a low-cost comonomer, which is nontoxic and allows for this copolymer to be
FIGURE 2.24 Polymerization of EVA.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.