Page 96 - Handbook of Plastics Technologies
P. 96
THERMOPLASTICS
2.36 CHAPTER 2
2.2.18 Polyarylether Ketones
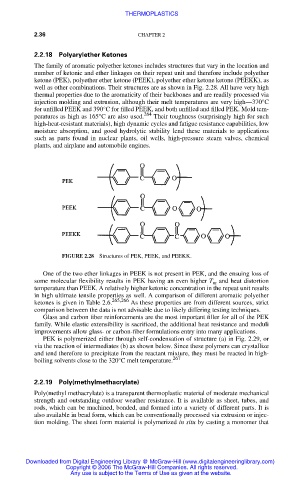
The family of aromatic polyether ketones includes structures that vary in the location and
number of ketonic and ether linkages on their repeat unit and therefore include polyether
ketone (PEK), polyether ether ketone (PEEK), polyether ether ketone ketone (PEEKK), as
well as other combinations. Their structures are as shown in Fig. 2.28. All have very high
thermal properties due to the aromaticity of their backbones and are readily processed via
injection molding and extrusion, although their melt temperatures are very high—370°C
for unfilled PEEK and 390°C for filled PEEK, and both unfilled and filled PEK. Mold tem-
264
peratures as high as 165°C are also used. Their toughness (surprisingly high for such
high-heat-resistant materials), high dynamic cycles and fatigue resistance capabilities, low
moisture absorption, and good hydrolytic stability lend these materials to applications
such as parts found in nuclear plants, oil wells, high-pressure steam valves, chemical
plants, and airplane and automobile engines.
FIGURE 2.28 Structures of PEK, PEEK, and PEEKK.
One of the two ether linkages in PEEK is not present in PEK, and the ensuing loss of
some molecular flexibility results in PEK having an even higher T and heat distortion
m
temperature than PEEK. A relatively higher ketonic concentration in the repeat unit results
in high ultimate tensile properties as well. A comparison of different aromatic polyether
265,266
ketones is given in Table 2.6. As these properties are from different sources, strict
comparison between the data is not advisable due to likely differing testing techniques.
Glass and carbon fiber reinforcements are the most important filler for all of the PEK
family. While elastic extensibility is sacrificed, the additional heat resistance and moduli
improvements allow glass- or carbon-fiber formulations entry into many applications.
PEK is polymerized either through self-condensation of structure (a) in Fig. 2.29, or
via the reaction of intermediates (b) as shown below. Since these polymers can crystallize
and tend therefore to precipitate from the reactant mixture, they must be reacted in high-
267
boiling solvents close to the 320°C melt temperature.
2.2.19 Poly(methylmethacrylate)
Poly(methyl methacrylate) is a transparent thermoplastic material of moderate mechanical
strength and outstanding outdoor weather resistance. It is available as sheet, tubes, and
rods, which can be machined, bonded, and formed into a variety of different parts. It is
also available in bead form, which can be conventionally processed via extrusion or injec-
tion molding. The sheet form material is polymerized in situ by casting a monomer that
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.