Page 98 - Handbook of Plastics Technologies
P. 98
THERMOPLASTICS
2.38 CHAPTER 2
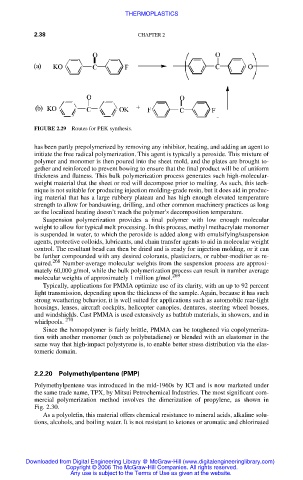
FIGURE 2.29 Routes for PEK synthesis.
has been partly prepolymerized by removing any inhibitor, heating, and adding an agent to
initiate the free radical polymerization. This agent is typically a peroxide. This mixture of
polymer and monomer is then poured into the sheet mold, and the plates are brought to-
gether and reinforced to prevent bowing to ensure that the final product will be of uniform
thickness and flatness. This bulk polymerization process generates such high-molecular-
weight material that the sheet or rod will decompose prior to melting. As such, this tech-
nique is not suitable for producing injection molding-grade resin, but it does aid in produc-
ing material that has a large rubbery plateau and has high enough elevated temperature
strength to allow for bandsawing, drilling, and other common machinery practices as long
as the localized heating doesn’t reach the polymer’s decomposition temperature.
Suspension polymerization provides a final polymer with low enough molecular
weight to allow for typical melt processing. In this process, methyl methacrylate monomer
is suspended in water, to which the peroxide is added along with emulsifying/suspension
agents, protective colloids, lubricants, and chain transfer agents to aid in molecular weight
control. The resultant bead can then be dried and is ready for injection molding, or it can
be further compounded with any desired colorants, plasticizers, or rubber-modifier as re-
268
quired. Number-average molecular weights from the suspension process are approxi-
mately 60,000 g/mol, while the bulk polymerization process can result in number average
269
molecular weights of approximately 1 million g/mol.
Typically, applications for PMMA optimize use of its clarity, with an up to 92 percent
light transmission, depending upon the thickness of the sample. Again, because it has such
strong weathering behavior, it is well suited for applications such as automobile rear-light
housings, lenses, aircraft cockpits, helicopter canopies, dentures, steering wheel bosses,
and windshields. Cast PMMA is used extensively as bathtub materials, in showers, and in
whirlpools. 270
Since the homopolymer is fairly brittle, PMMA can be toughened via copolymeriza-
tion with another monomer (such as polybutadiene) or blended with an elastomer in the
same way that high-impact polystyrene is, to enable better stress distribution via the elas-
tomeric domain.
2.2.20 Polymethylpentene (PMP)
Polymethylpentene was introduced in the mid-1960s by ICI and is now marketed under
the same trade name, TPX, by Mitsui Petrochemical Industries. The most significant com-
mercial polymerization method involves the dimerization of propylene, as shown in
Fig. 2.30.
As a polyolefin, this material offers chemical resistance to mineral acids, alkaline solu-
tions, alcohols, and boiling water. It is not resistant to ketones or aromatic and chlorinated
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.