Page 84 - High Power Laser Handbook
P. 84
54 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Chemical Lasers 55
0.6
Cold
Hot
0.5
0.4
Nascent fraction 0.3
0.2
0.1
0
0 1 2 3 4 5 6 7 8 9
Vibrational level
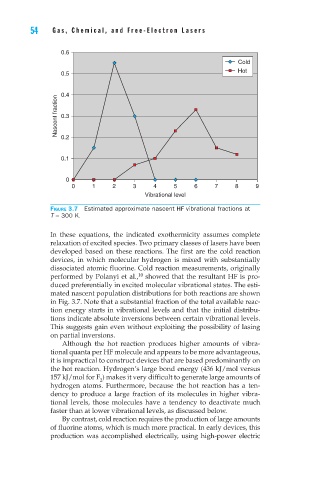
Figure 3.7 Estimated approximate nascent HF vibrational fractions at
T = 300 K.
In these equations, the indicated exothermicity assumes complete
relaxation of excited species. Two primary classes of lasers have been
developed based on these reactions. The first are the cold reaction
devices, in which molecular hydrogen is mixed with substantially
dissociated atomic fluorine. Cold reaction measurements, originally
performed by Polanyi et al., showed that the resultant HF is pro-
10
duced preferentially in excited molecular vibrational states. The esti-
mated nascent population distributions for both reactions are shown
in Fig. 3.7. Note that a substantial fraction of the total available reac-
tion energy starts in vibrational levels and that the initial distribu-
tions indicate absolute inversions between certain vibrational levels.
This suggests gain even without exploiting the possibility of lasing
on partial inversions.
Although the hot reaction produces higher amounts of vibra-
tional quanta per HF molecule and appears to be more advantageous,
it is impractical to construct devices that are based predominantly on
the hot reaction. Hydrogen’s large bond energy (436 kJ/mol versus
157 kJ/mol for F ) makes it very difficult to generate large amounts of
2
hydrogen atoms. Furthermore, because the hot reaction has a ten-
dency to produce a large fraction of its molecules in higher vibra-
tional levels, those molecules have a tendency to deactivate much
faster than at lower vibrational levels, as discussed below.
By contrast, cold reaction requires the production of large amounts
of fluorine atoms, which is much more practical. In early devices, this
production was accomplished electrically, using high-power electric