Page 85 - High Power Laser Handbook
P. 85
54 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Chemical Lasers 55
100
80
Fraction dissociated 60
40
20 3 psia
10 psia
30 psia
0
800 1000 1200 1400 1600 1800 2000 2200
Temperature (K)
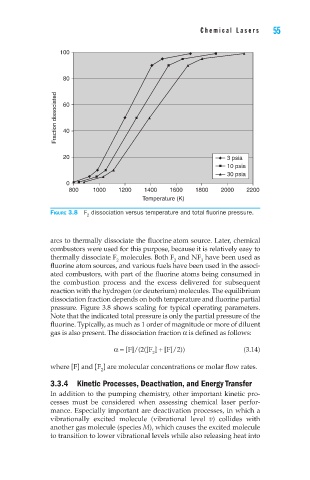
Figure 3.8 F dissociation versus temperature and total fluorine pressure.
2
arcs to thermally dissociate the fluorine atom source. Later, chemical
combustors were used for this purpose, because it is relatively easy to
thermally dissociate F molecules. Both F and NF have been used as
3
2
2
fluorine atom sources, and various fuels have been used in the associ-
ated combustors, with part of the fluorine atoms being consumed in
the combustion process and the excess delivered for subsequent
reaction with the hydrogen (or deuterium) molecules. The equilibrium
dissociation fraction depends on both temperature and fluorine partial
pressure. Figure 3.8 shows scaling for typical operating parameters.
Note that the indicated total pressure is only the partial pressure of the
fluorine. Typically, as much as 1 order of magnitude or more of diluent
gas is also present. The dissociation fraction α is defined as follows:
α = [F]/(2([F ] + [F]/2)) (3.14)
2
where [F] and [F ] are molecular concentrations or molar flow rates.
2
3.3.4 Kinetic Processes, Deactivation, and Energy Transfer
In addition to the pumping chemistry, other important kinetic pro-
cesses must be considered when assessing chemical laser perfor-
mance. Especially important are deactivation processes, in which a
vibrationally excited molecule (vibrational level v) collides with
another gas molecule (species M), which causes the excited molecule
to transition to lower vibrational levels while also releasing heat into