Page 86 - High Power Laser Handbook
P. 86
56 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Chemical Lasers 57
the flow. This is referred to as the vibrational to translational energy
(VT) processes.
HF(v) + M → HF(v – m) + M + ∆Q (3.15)
The most significant deactivating species in HF and DF devices
are typically the hydrogen halides themselves, including both the las-
ing species and the combustor combustion products. Measurements
of kinetic rates associated with these processes have indicated that the
deactivation rate has between a second- and third-power dependence
on v. In addition, a large increase in the deactivation of HF(v) for
vibrational levels greater than or equal to 3 was observed for hydro-
gen atom deactivation. These characteristics favor the cold reaction
(F + H ), discussed earlier, over the hot one (H + F ). In addition, it was
2
2
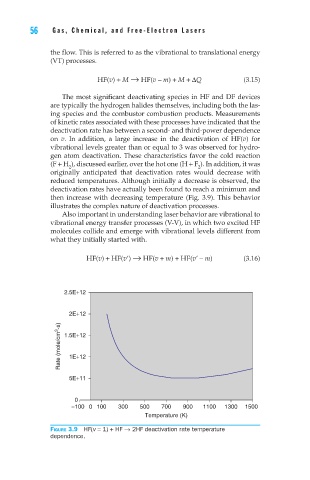
originally anticipated that deactivation rates would decrease with
reduced temperatures. Although initially a decrease is observed, the
deactivation rates have actually been found to reach a minimum and
then increase with decreasing temperature (Fig. 3.9). This behavior
illustrates the complex nature of deactivation processes.
Also important in understanding laser behavior are vibrational to
vibrational energy transfer processes (V-V), in which two excited HF
molecules collide and emerge with vibrational levels different from
what they initially started with.
HF(v) + HF(v’) → HF(v + m) + HF(v’ – m) (3.16)
2.5E+12
2E+12
Rate (mole/cm 3 -s) 1.5E+12
1E+12
5E+11
0
−100 0 100 300 500 700 900 1100 1300 1500
Temperature (K)
Figure 3.9 HF(v = 1) + HF → 2HF deactivation rate temperature
dependence.