Page 134 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 134
Electrolytes 11 1
electronic hole conductivity of SrCe0.95Yb0.0503 in dry air is 0.01 S/cm at 800°C.
When this oxide is exposed to humidified air, protons as charge carriers are
generated in the lattice by the following reaction
H2 + 2h' = 2H'
and the electrical conductivity is increased due to proton conduction. The
conductivity of SrCe0.95Yb0.0503 in humidified atmospheres was around
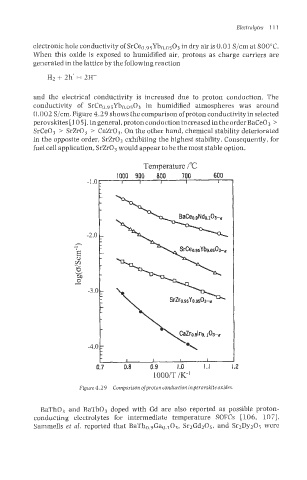
0.002 S/cm. Figure 4.29 shows the comparison ofproton conductivity in selected
perovskites [ 1051. In general, proton conduction increased in the order BaCe03 >
SlrCe03 > SrZr0, > CaZrO,. On the other hand, chemical stability deteriorated
in the opposite order, SrZr03 exhibiting the highest stability. Consequently, for
fuel cell application, SrZrO, would appear to be the most stable option.
/"C
Temperature /"C
Temperature
800
900
1000 900 800 700 600
600
700
1000
-1 .o F I I I I I I I I I I 1 1
-1 .o F
-2.0
n
4
i3
0
m
-5
v
M
0
-3.0
-4.0
I I L I 1
0.7 0.8 0.9 I .o 1.1 I .2
1000/T /IC1
Figure 4.29 Comparison ofgroton conduction inperovskite oxides.
EaTh03 and BaTb03 doped with Gd are also reported as possible proton-
conducting electrolytes for intermediate temperature SOFCs [106, 1071.
Samrnells et al. reported that BaTho.gGao,103, Sr2Gd20s, and Sr2Dy205 were