Page 133 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 133
110 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
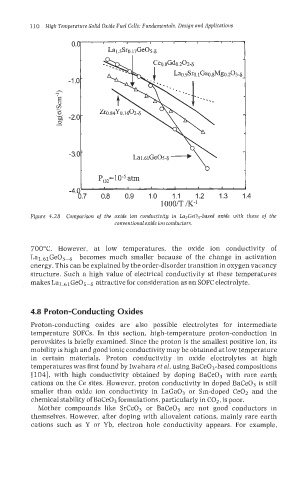
P,=10-5 atm
700°C. However, at low temperatures, the oxide ion conductivity of
Lal.61Ge05-8 becomes much smaller because of the change in activation
energy. This can be explained by the order-disorder transition in oxygen vacancy
structure. Such a high value of electrical conductivity at these temperatures
makes Lal.61Ge05-h attractive for consideration as an SOFC electrolyte.
4.8 Proton-Conducting Oxides
Proton-conducting oxides are also possible electrolytes for intermediate
temperature SOFCs. In this section, high-temperature proton-conduction in
perovskites is briefly examined. Since the proton is the smallest positive ion, its
mobility is high and good ionic conductivity may be obtained at low temperature
in certain materials. Proton conductivity in oxide electrolytes at high
temperatures was first found by Iwahara et al. using BaCe03-based compositions
[104], with high conductivity obtained by doping BaCeOs with rare earth
cations on the Ce sites. However, proton conductivity in doped BaCe03 is still
smaller than oxide ion conductivity in LaGa03 or Sm-doped Ce02 and the
chemical stability of BaCe03 formulations, particularly in C02, is poor.
Mother compounds like SrCe03 or BaCeOs are not good conductors in
themselves. However, after doping with aliovalent cations, mainly rare earth
cations such as Y or Yb, electron hole conductivity appears. For example,