Page 131 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 131
108 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
0.0 1 I I
-1.0 -
h
9
:: -2.0 -
3
v
30
0
c
-3.0 -
:
-4.0
0.8 0.9 1.0 1.1 1.2
1000 /-I-
K-1
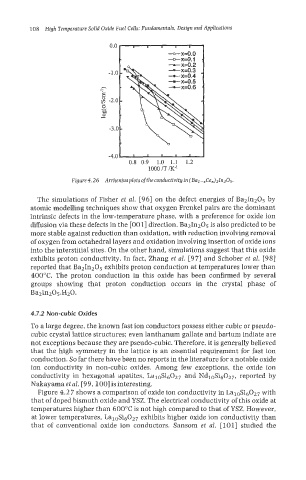
Figure4.26 Arrheniusplotsof the conductivity in (Bal-,CeX),ln2O5.
The simulations of Fisher et al. [96] on the defect energies of Ba21n205 by
atomic modelling techniques show that oxygen Frenkel pairs are the dominant
intrinsic defects in the low-temperature phase, with a preference for oxide ion
diffusion via these defects in the [OOl] direction. BazIn20j is also predicted to be
more stable against reduction than oxidation, with reduction involving removal
of oxygen from octahedral layers and oxidation involving insertion of oxide ions
into the interstitial sites. On the other hand, simulations suggest that this oxide
exhibits proton conductivity. In fact, Zhang et al. [97] and Schober et al. [98]
reported that Ba21n205 exhibits proton conduction at temperatures lower than
400°C. The proton conduction in this oxide has been confirmed by several
groups showing that proton conduction occurs in the crystal phase of
Ea2In2O5.H20.
4.7.2 Non-cubic Oxides
To a large degree, the known fast ion conductors possess either cubic or pseudo-
cubic crystal lattice structures; even lanthanum gallate and barium indiate are
not exceptions because they are pseudo-cubic. Therefore, it is generally believed
that the high symmetry in the lattice is an essential requirement for fast ion
conduction. So far there have been no reports in the literature for a notable oxide
ion conductivity in non-cubic oxides. Among few exceptions, the oxide ion
conductivity in hexagonal apatites, LaloSi6027 and Nd10Si602,, reported by
Nakayamaetal. [99,100] isinteresting.
Figure 4.27 shows a comparison of oxide ion conductivity in LaloSi6027 with
that of doped bismuth oxide and YSZ. The electrical conductivity of this oxide at
temperatures higher than 600°C is not high compared to that of YSZ. However,
at lower temperatures, Lal&,02 exhibits higher oxide ion conductivity than
that of conventional oxide ion conductors. Sansom et al. [loll studied the