Page 126 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 126
Electrolytes 103
Yokokawa et al. estimated the electrolyte efficiency of LSGM used as the
electrolyte in an SOFC [78]. Electrolyte efficiency was given by a function of fuel
utilisation and internal resistance [ 761. When the thickness of the electrolyte was
too small, chemical leakage of oxygen due to the electronic conduction became
significant and the extra fuel consumption resulted in decreased electrolyte
efficiency. On the other hand, with increasing thickness of electrolyte, internal
resistance of the cell increased also resulting in decreased electrolyte efficiency.
Consequently, an optimum thickness exists for each electrolyte material at a given
temperature and current density. For example YSZ operating at 700°C has an
optimum thickness of about 10 pm. The benefit of LSGM is that its highest
efficiency with a thickness near 5 pm is achieved around 450°C. Thus LSGM
appears to be the best electrolyte discovered so far to operate at low temperatures.
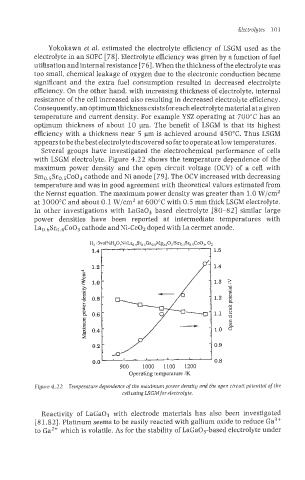
Several groups have investigated the electrochemical performance of cells
with LSGM electrolyte. Figure 4.22 shows the temperature dependence of the
maximum power density and the open circuit voltage (OCV) of a cell with
Smo.5Sro.5C003 cathode and Ni anode [79]. The OCV increased with decreasing
temperature and was in good agreement with theoretical values estimated from
the Nernst equation. The maximum power density was greater than 1.0 W/cm2
at 1000°C and about 0.1 W/cm2 at 600°C with 0.5 mm thick LSGM electrolyte.
in other investigatfons with LaGaQ3 based electrolyte [80-821 similar large
power densities have been reported at intermediate temperatures with
Lao~6Sro.4C003 cathode and Ni-CeOl doped with La cermet anode.
H,-3vol%H~O,Ni/Lao,~Sro~,Gao,~Mgo~~O~~Smo~~Sr~,~CoO~,
0,
1.5
Operating temperature /K
Figure 4.22 Temperature dependence of the maximum power density nnd the open circuit potential of the
cell using LSGMfar electroIyte.
Reactivity of LaGa03 with electrode materials has also been investigated
[81,82]. Platinum seems to be easily reacted with gallium oxide to reduce Ga3+
to Ga2+ which is volatile. As for the stability of LaGa03-based electrolyte under