Page 124 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 124
Electrolytes 101
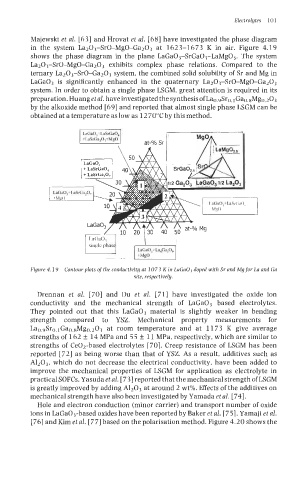
Majewski et al. [63] and Hrovat et al. [68] have investigated the phase diagram
in the system La203-Sr0-Mg0-Ga203 at 1623-1673 K in air. Figure 4.19
shows the phase diagram in the plane LaGa03-SrGa03-LaMg03. The system
La203-Sr0-Mg0-Ga203 exhibits complex phase relations. Compared to the
ternary La203-Sr0-Ga203 system, the combined solid solubility of Sr and Mg in
LaGa03 is significantly enhanced in the quaternary La203-Sr0-Mg0-Ga203
system. In order to obtain a single phase LSGM, great attention is required in its
preparation. Huang et al. have investigated the synthesis 0fLa~.~Sr~.~Ga~.~Mg~.~0~
by the alkoxide method [69] and reported that almost single phase LSGM can be
obtained at a temperature as low as 12 70°C by this method.
LaGaO,+LaSrGaO,
+LaSffia,O,+MgO ____
\ n 1 I LaMgO,,
..___
L
-
LaGaO, +I
Figure 4.1 9 Contour plots of the conductivity at 1073 Kin LaGaOs doped with Sr and Mg for La and Ga
site. respectively.
Drennan et al. [70] and Du et al. [71] have investigated the oxide ion
conductivity and the mechanical strength of LaGa03 based electrolytes.
They pointed out that this LaGa03 material is slightly weaker in bending
strength compared to YSZ. Mechanical property measurements for
Lao.9Sro.lGao.8Mgo,203 at room temperature and at 11 73 K give average
strengths of 162 f 14 MPa and 55 f 11 MPa, respectively, which are similar to
strengths of Ce02-based electrolytes [70]. Creep resistance of LSGM has been
reported [72] as being worse than that of YSZ. As a result, additives such as
A1203, which do not decrease the electrical conductivity, have been added to
improve the mechanical properties of LSGM for application as electrolyte in
practical SOFCs. Yasuda et al. [73] reported that the mechanical strength ofLSGM
is greatly improved by adding A1203 at around 2 wt%. Effects of the additives on
mechanical strength have also been investigated by Yamada et al. [74].
Hole and electron conduction (minor carrier) and transport number of oxide
ions in LaGa03-based oxides have been reported by Baker et al. [75], Yamaji et al.
[76] and Kim et al. [77] based on the polarisation method. Figure 4.20 shows the