Page 120 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 120
Electrolytes 97
SOFCs [52]. Only few perovskites are purely ionic in their conduction behaviour.
Properties of selected ionic-conducting perovskites are discussed below.
4.6.7 kiAI03
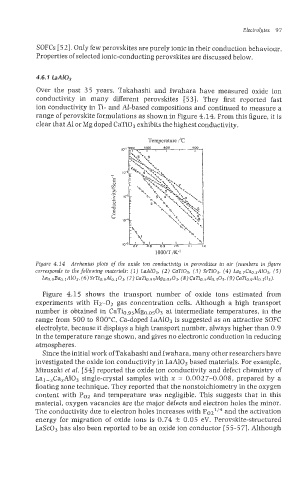
Over the past 35 years, Takahashi and Iwahara have measured oxide ion
conductivity in many different perovskites [53]. They first reported fast
ion conductivity in Ti- and AI-based compositions and continued to measure a
range of perovsltite formulations as shown in Figure 4.14. From this figure, it is
clear that A1 or Mg doped CaTi03 exhibits the highest conductivity.
Temperature !“C
1 OOO/T /K-I
Figure 4.14 Arrhenius plots of the oxide ion conductivity in perowkites in air (numbers in jgure
corresponds to the foZlowing materials: (I) LnAI03, (2) CaTi03, (3) SrTi03, (4) Lu~.~CUO.~A~O~, (5)
(8)
La0.gBao.lAI03, (6) SrTio.~Al0.103~ (7) Cari~,~~Mg~,~Kb CaTio.jAb.io3r (9) CaTio,9AI0..3031.
Figure 4.15 shows the transport number of oxide ions estimated from
experiments with H2-02 gas concentration cells. Although a high transport
number is obtained in CaTi0,95Mg0.0503 at intermediate temperatures, in the
range from 500 to 800°C Ca-doped LaA103 is suggested as an attractive SOFC
electrolyte, because it displays a high transport number, always higher than 0.9
in the temperature range shown, and gives no electronic conduction in reducing
atmospheres .
Since the initial work of Takahashi and Iwahara, many other researchers have
investigated the oxide ion conductivity in LaA103 based materials. For example,
Mizusaki et al. [54] reported the oxide ion conductivity and defect chemistry of
Lal-,Ca,A103 single-crystal samples with x = 0.002 7-0.008, prepared by a
floating zone technique. They reported that the nonstoichiometry in the oxygen
content with Poz and temperature was negligible. This suggests that in this
material, oxygen vacancies are the major defects and electron holes the minor.
The conductivity due to electron holes increases with PO2ll4 and the activation
energy for migration of oxide ions is 0.74 5 0.05 eV. Perovskite-structured
LaSc03 has also been reported to be an oxide ion conductor [55-571. Although