Page 116 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 116
Electrolytes 93
- I-
= 2.0 0.5
c I
0.4
ai
Y
0.3 2
{:
0.1
'-
0.10 0.11 0.12
Rddiiis of OOD3nt Cation / nm
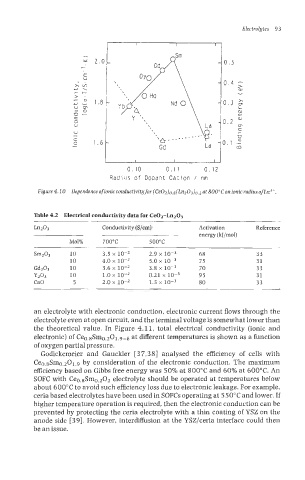
Figure 4.10 Dependence of ionic conductivity for (Ce02)o.8(Ln203)o.2 at 800"Con ionic radius of Ln3'.
Table 4.2 Electrical conductivity data for Ce02-LnzOs
Conductivity (S/cm) Activation Reference
energy (kJ/mol)
Mol% 700°C 500°C
Sm203 10 3.5 x 10-2 2.9 x 10-3 68 33
10 4.0 x 5.0 x 10-3 75 31
Gd203 10 3.6 x 3.8 x 10-3 70 33
y203 10 1.0 x 10-2 0.21 x 10-3 95 31
CaO 5 2.0 x 10-2 1.5 x 10-3 80 33
an electrolyte with electronic conduction, electronic current flows through the
electrolyte even at open circuit, and the terminal voltage is somewhat lower than
the theoretical value. In Figure 4.1 1, total electrical conductivity (ionic and
at
electronic) of Ceo.~Smo.z01,9~8 different temperatures is shown as a function
of oxygen partial pressure.
Godickemeier and Gauckler [3 7,381 analysed the efficiency of cells with
Ceo.8Smo.201.9 by consideration of the electronic conduction. The maximum
efficiency based on Gibbs free energy was 50% at 800°C and 60% at 600°C. An
SOFC with Ceo.8Smo.202 electrolyte should be operated at temperatures below
about 600°C to avoid such efficiency loss due to electronic leakage. For example,
ceria based electrolytes have been used in SOFCs operating at 550°C and lower. If
higher temperature operation is required, then the electronic conduction can be
prevented by protecting the ceria electrolyte with a thin coating of YSZ on the
anode side [39]. However, interdiffusion at the YSZ/ceria interface could then
be an issue.