Page 113 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 113
90 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
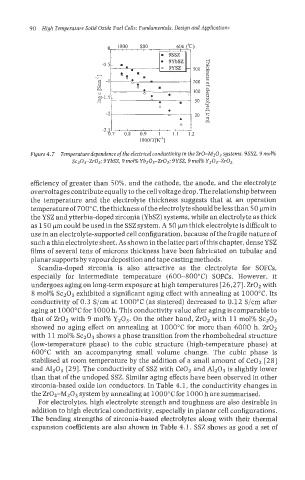
Figure 4.7 Temperature dependence of the electrical conductivity in the ZrO-M2O3 systems. 9SSZ. 9 mol%
Sc203-Zr02: 9YbSZ. 9 mol% Yb203-Zr02; 9YSZ, 9 mol% Y203-Zr-02.
efficiency of greater than 50%, and the cathode, the anode, and the electrolyte
overvoltages contribute equally to the cell voltage drop. The relationship between
the temperature and the electrolyte thickness suggests that at an operation
temperature of 700°C, the thickness of the electrolyte should be less than 50 pm in
the YSZ and ytterbia-doped zirconia (YbSZ) systems, while an electrolyte as thick
as 150 pm could be used in the SSZ system. A 50 pm thick electrolyte is difficult to
use in an electrolyte-supported cell configuration, because of the fragile nature of
such a thin electrolyte sheet. As shown in the latter part of this chapter, dense YSZ
films of several tens of microns thickness have been fabricated on tubular and
planar supports by vapour deposition and tape casting methods.
Scandia-doped zirconia is also attractive as the electrolyte for SOFCs,
especially for intermediate temperature (600-800°C) SOFCs. However, it
undergoes aging on long-term exposure at high temperatures [26,2 71. ZrOz with
8 mol% Sc203 exhibited a significant aging effect with annealing at 1000°C. Its
conductivity of 0.3 S/cm at 1000°C (as sintered) decreased to 0.12 S/cm after
aging at 1000°C for 1000 h. This conductivity value after aging is comparable to
that of Zr02 with 9 mol% Y2O3. On the other hand, ZrO2 with 11 mol% Sc203
showed no aging effect on annealing at 1000°C for more than 6000 h. Zr02
with 11 mol% ScZO3 shows a phase transition from the rhombohedral structure
(low-temperature phase) to the cubic structure (high-temperature phase) at
600°C with an accompanying small volume change. The cubic phase is
stabilised at room temperature by the addition of a small amount of Ce02 [28]
and A1203 [29]. The conductivity of SSZ with Ce02 and A1203 is slightly lower
than that of the undoped SSZ. Similar aging effects have been observed in other
zirconia-based oxide ion conductors. In Table 4.1, the conductivity changes in
the ZrOz-M2O3 system by annealing at 1000°C for 1000 hare summarised.
For electrolytes, high electrolyte strength and toughness are also desirable in
addition to high electrical conductivity, especially in planar cell configurations.
The bending strengths of zirconia-based electrolytes along with their thermal
expansion coefficients are also shown in Table 4.1. SSZ shows as good a set of