Page 111 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 111
88 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and AppIications
At higher temperatures the complex (V;-Mzr'). dissociates completely to free V;
and Mz:. The concentration of V; is independent of the temperature and equal to
the total concentration of dopant M3+. Therefore, the migration enthalpy, E,,
could be estimated from the slope of the temperature dependence for conduction
in the higher temperature range. The association enthalpy could be calculated
from the difference in the slopes at the lower and the higher temperature ranges.
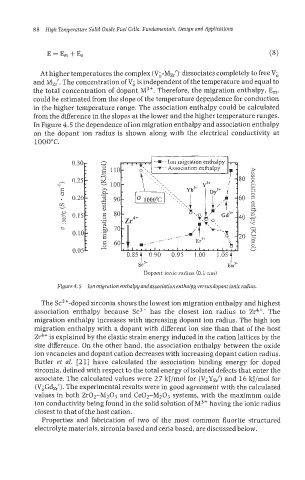
In Figure 4.5 the dependence of ion migration enthalpy and association enthalpy
on the dopant ion radius is shown along with the electrical conductivity at
0.30-
- 0.25-
'I!
. 0.20-
3
0.15;
s
0
0.10-
0.05-
Figure 4.5 Ion migration enthalpy andassociation enthalpy versus dopant ionic radius.
The Sc"-doped zirconia shows the lowest ion migration enthalpy and highest
association enthalpy because Sc3+ has the closest ion radius to Zr4+. The
migration enthalpy increases with increasing dopant ion radius. The high ion
migration enthalpy with a dopant with different ion size than that of the host
Zr4+ is explained by the elastic strain energy induced in the cation lattices by the
size difference. On the other hand, the association enthalpy between the oxide
ion vacancies and dopant cation decreases with increasing dopant cation radius.
Butler et al. [21] have calculated the association binding energy for doped
zirconia, defined with respect to the total energy of isolated defects that enter the
associate. The calculated values were 2 7 kJ/mol for (V;Yz,') and 16 kJ/mol for
(ViGd,,'). The experimental results were in good agreement with the calculated
values in both Zr02-M203 and Ce02-M203 systems, with the maximum oxide
ion conductivity being found in the solid solution of M3+ having the ionic radius
closest to that of the host cation.
Properties and fabrication of two of the most common fluorite structured
electrolyte materials, zirconia based and ceria based, are discussed below.